Batch Manufacturing Record (BMR) – What It Is and Why It Matters
In pharmaceuticals, food, supplements, cosmetics, and other regulated industries, the Batch Manufacturing Record (BMR) is the backbone of both operational discipline and regulatory compliance. A BMR is more than paperwork—it’s the proof your batch was made exactly as approved, in accordance with Good Manufacturing Practice (GMP), internal SOPs, and standards such as 21 CFR Part 211, ISO 13485, and EU Annex 11.
What Does a Batch Manufacturing Record (BMR) Include?
A compliant BMR documents all critical details for each batch:
- Batch number and product ID
- Raw material lots, suppliers, and quantities
- Process steps, in-process controls, and operator sign-offs
- Equipment, cleaning, and calibration logs
- Direct data capture from scales, scanners, and automated devices
- Deviation reports, rework, and CAPA actions
- Final yield, reconciliation, and QA release signature
The BMR Lifecycle: From Creation to Audit
- Drafting: Authored from MMR, SOPs, and process specs
- Approval: QA review for GMP compliance and completeness
- Issuance: Controlled copies for batch execution (paper or digital)
- Execution: Operators record data, sign-offs, deviations
- Review: QA reviews records for completeness and compliance
- Archival: Final BMR archived for required retention period and audits
Compliance, Audit, and Common Pitfalls
Each BMR must be complete, accurate, and reviewed. Regulatory authorities such as the FDA and EMA regularly cite manufacturers for:
- Missing or incomplete entries and sign-offs
- Use of outdated or uncontrolled forms
- Illegible or ambiguous data entries
- Unreported deviations or undocumented rework
- Data integrity violations (altered, backdated, or inconsistent records)
- Inadequate review or lack of QA signatures
“Since implementing a digital batch record system, our batch reviews take less than 30 minutes—with zero missed deviations and full traceability to every operator and ingredient.”
— Director of QA, Pharmaceutical Manufacturer
Source: FDA, EMA, WHO inspection reports.
Case Study: How BMR Practices Affect Audit Outcomes
In a recent FDA inspection, a nutraceutical manufacturer was cited for using an uncontrolled BMR—resulting in a warning letter and recall. By contrast, a site using electronic BMR reduced review time by 80% and passed audits with no major findings.
Paper vs. Electronic BMR (eBMR) vs. Hybrid Systems
| System | Pros | Cons |
|---|---|---|
| Paper | Familiar, low-tech, no upfront cost | Manual errors, slow review, difficult audits, data integrity risks |
| eBMR | Real-time capture, automated checks, audit-ready, faster review | Requires validation, investment, and user training |
| Hybrid | Stepwise migration, familiar workflows | Manual steps remain, risk of gaps, harder to enforce compliance |
Real-World Example: Electronic Batch Manufacturing Record (eBMR) Sample Page
Below is an excerpt from an actual Electronic Batch Manufacturing Record (eBMR) report generated using SG Systems’ V5 Traceability platform. This is just one page from a much larger report, which can include dozens or even hundreds of pages covering the entire batch lifecycle.
In this sample page, you can see key dispensary activities for a single batch—each row captures who performed the action, on which terminal, at what time, the material involved, and the outcome (e.g., PASS, GAP, deviation raised). The log includes checklists, deviation handling, line checks for hazards and allergens, and a summary of all weighing and dispensing events. This level of granularity, traceability, and operator accountability is what regulators expect for true GMP compliance.
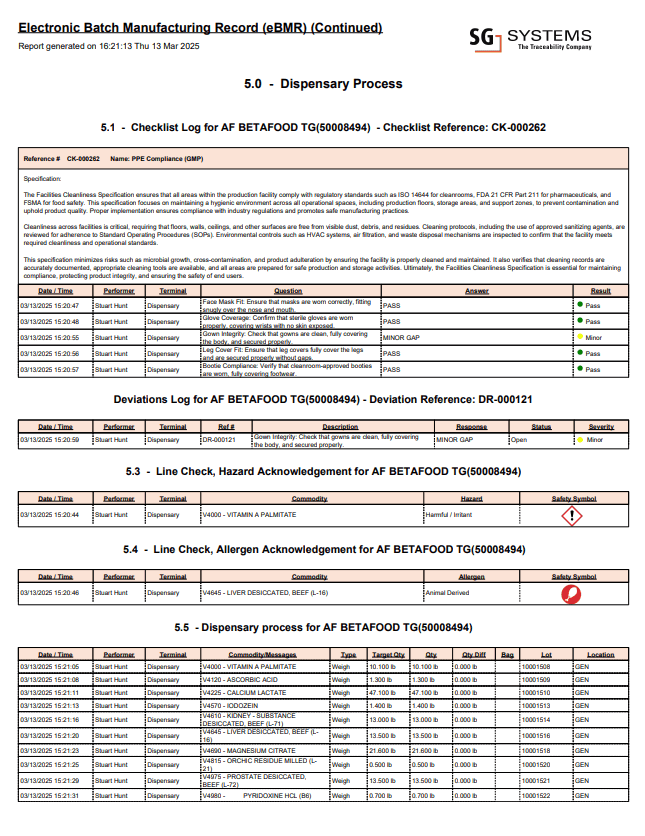
Sample eBMR report page generated from live data on the shop floor. This is one page from a multi-page batch record, showing detailed dispensing activities, deviation logs, line checks, and operator sign-offs—all fully traceable and audit-ready.
Glossary of Batch Record Terms
- BMR
- Batch Manufacturing Record – documents production for each batch
- eBMR
- Electronic Batch Manufacturing Record – digital version with enforced controls
- MMR
- Master Manufacturing Record – template for creating BMRs
- DHR
- Device History Record – batch record equivalent in medical devices
- SOP
- Standard Operating Procedure – controlled instruction for manufacturing steps
- CAPA
- Corrective and Preventive Action – managing deviations and root cause analysis
Frequently Asked Questions
What is a Batch Manufacturing Record?
A Batch Manufacturing Record (BMR) is the complete, verified documentation of each batch’s production, test, and release—required for compliance and quality management.
What’s the difference between BMR and eBMR?
BMR is traditionally paper-based. eBMR is a secure, digital version that captures data directly from shop floor devices, enforces workflows, and secures signatures.
Why do BMRs fail regulatory audits?
Common reasons include missing or incomplete entries, use of outdated forms, illegible data, unreported deviations, and poor data integrity practices.
References
-
FDA. Guidance for Industry: Quality Systems Approach to Pharmaceutical CGMP Regulations.
-
FDA. Data Integrity and Compliance With Drug CGMP – Guidance for Industry.
-
EMA. Annex 11 – Computerised Systems.
-
WHO. Technical Report Series No. 996 – Good Data and Record Management Practices.
-
ISO. ISO 9001:2015 – Quality Management Systems.
-
PIC/S. Good Practices for Data Management and Integrity in Regulated GMP/GDP Environments.



