Digital Transformation: Electronic Batch Record Pharma Solutions for GMP Compliance
Electronic batch record pharma systems are redefining operational control, traceability, and regulatory compliance in the pharmaceutical sector. As global GMP standards and data integrity expectations continue to rise, pharmaceutical manufacturers are moving rapidly from paper-based records to electronic batch record pharma technology. This shift delivers real-time audit readiness, live data capture, and a defensible record of every production step.
What Is an Electronic Batch Record Pharma Platform?
An electronic batch record pharma solution digitizes every aspect of batch documentation—recording materials, processes, operator actions, equipment use, and results directly from the manufacturing environment. Unlike manual or hybrid paper processes, electronic batch record pharma platforms enforce step-by-step compliance, apply electronic signatures, and deliver a complete, audit-ready trail for inspectors. Learn more from the FDA 21 CFR Part 11 guidance and the EMA Annex 11 guideline.
- Real-time data capture and validation
- Automated enforcement of SOPs and batch instructions
- Electronic signatures with user authentication
- Automated deviation capture and CAPA management
- End-to-end audit trails and version control
- Integration with pharma MES, ERP, and QMS systems
Paper vs. Electronic Batch Record Pharma Systems
Paper batch records remain technically legal in pharma, but electronic batch record pharma solutions are quickly becoming the industry standard. The reasons are clear: paper is prone to error, illegibility, and delays, while electronic batch record pharma technology enables live compliance and instant traceability.
| Function | Paper Record | Electronic Batch Record Pharma |
|---|---|---|
| Format | Handwritten/manual | Digital, validated, compliant |
| Audit Trail | Manual, delayed, error-prone | Automated, time-stamped, audit-ready |
| Signatures | Wet ink, often incomplete | Electronic, enforced, 21 CFR Part 11 compliant |
| Deviation Handling | Manual, retrospective | Real-time, automated, escalated |
| Batch Review | Slow, error-prone, paper-based | Fast, digital, pharma-auditable |
Why the Pharmaceutical Industry Chooses Electronic Batch Record Pharma Solutions
Regulatory compliance in pharma is non-negotiable. Electronic batch record pharma technology addresses all critical requirements: data integrity, traceability, role-based controls, and defensible audit trails. Warning letters from agencies like the FDA consistently cite manual record-keeping failures. Electronic batch record pharma solutions automate compliance, reduce errors, and provide full visibility from raw material to finished product.
- Improved GMP and data integrity assurance
- Reduced batch release times and review costs
- Seamless integration with pharma enterprise systems
- Real-time deviation management and CAPA tracking
How Electronic Batch Record Pharma Platforms Work
Electronic batch record pharma platforms control every stage of pharmaceutical production. From dispensing to compounding, packaging to QA, every instruction, material, and signature is captured and validated digitally. Modern solutions like V5 from SG Systems Global enable enforceable workflows, real-time data validation, and effortless audit readiness in pharma.
- Automated SOP enforcement and electronic sign-offs
- Comprehensive traceability for each lot, batch, and ingredient
- Real-time data transfer to QMS, LIMS, and ERP
- Full support for medical device eDHR requirements
Electronic batch record pharma systems are now the baseline expectation for data integrity and GMP compliance in pharmaceutical inspections.
See an Example: Electronic Batch Record Pharma Sample Page
Below is an example excerpt from a multi-page electronic batch record pharma report, illustrating real-time data capture, digital sign-offs, and compliance checkpoints. This image is a single page from a full, validated eBR pharma report as generated by advanced digital solutions.
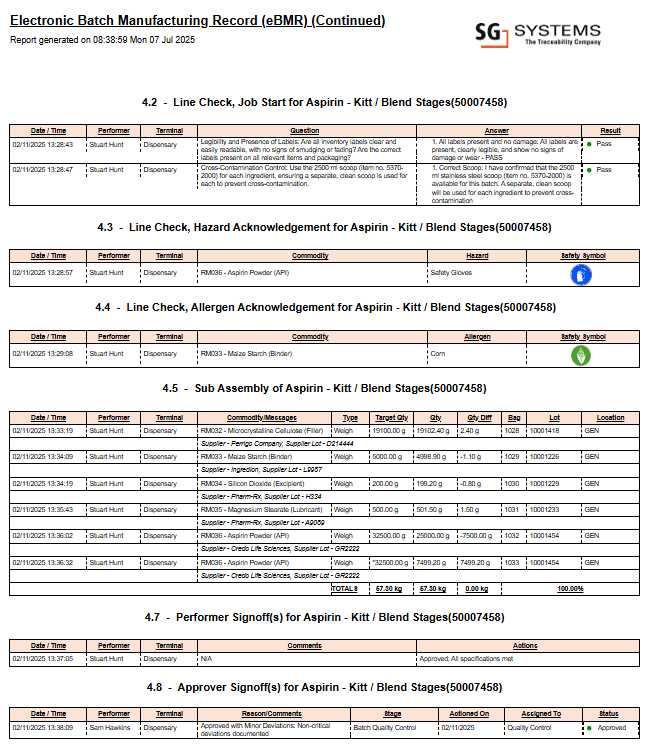
This image is an excerpt from a comprehensive multi-page electronic batch record pharma report generated by leading GMP digital systems.


