Electronic Batch Record Software: The Backbone of Digital Manufacturing Compliance
Electronic Batch Record Software is rapidly becoming a non-negotiable standard for manufacturers operating in highly regulated environments—pharmaceuticals, food & beverage, dietary supplements, cosmetics, and medical devices. With regulatory scrutiny and data integrity requirements rising globally, organizations are replacing outdated paper processes with digital batch record software to achieve real-time traceability, audit readiness, and total process control.
What Is Electronic Batch Record Software?
Electronic batch record software is an application platform designed to digitally capture, manage, and secure every step in the batch manufacturing process. It enables seamless documentation of raw materials, equipment, operator actions, process steps, deviations, and signatures—providing an end-to-end, audit-ready record for every batch. By automating data entry, enforcing SOPs, and supporting electronic signatures, modern EBR software ensures robust compliance with FDA 21 CFR Part 11, EMA Annex 11, ISPE GAMP 5, and global GFSI schemes.
- Real-time data capture with automated validation and exception management
- Step-by-step enforcement of batch instructions and Standard Operating Procedures (SOPs)
- Configurable electronic signatures with role-based security
- Automated deviation, NCR, and CAPA management
- Complete audit trails, version control, and revision histories
- Seamless integration with MES, QMS, ERP, LIMS, and supply chain platforms
- Data export and reporting for regulatory authorities and customers
The Shift from Paper to Electronic Batch Record Software
Many manufacturers still rely on paper-based batch records—acceptable, but increasingly risky. Paper records are vulnerable to errors, missing data, slow batch reviews, and failed audits. Regulatory bodies like the FDA and MHRA frequently issue warning letters for manual record-keeping failures. In contrast, electronic batch record software enables live data capture, instant traceability, and defensible digital records that streamline compliance with global requirements.
| Feature | Paper-Based Records | Electronic Batch Record Software |
|---|---|---|
| Data Integrity | Prone to loss and illegibility | Secure, validated, audit-ready |
| Traceability | Difficult, manual searching | Instant, searchable, end-to-end |
| Signatures | Wet ink, hard to authenticate | Electronic, compliant, fully tracked |
| Deviation Handling | Manual, slow, error-prone | Automated, real-time, CAPA integrated |
| Batch Review | Delayed, resource-intensive | Immediate, remote, streamlined |
| Compliance | Challenging for modern audits | Designed for FDA, EMA, ISO, GFSI, etc. |
Who Needs Electronic Batch Record Software?
Any manufacturer subject to Good Manufacturing Practice (GMP), ISO 9001, ISO 13485, BRCGS, SQF, GFSI, or similar regulatory frameworks benefits from electronic batch record software. Whether you make pharmaceuticals, food, supplements, or medical devices, digital batch record platforms ensure process consistency, product quality, and regulatory compliance—globally. Industry groups like the PDA and ISPE advocate for digitalization as the best practice for data integrity.
- Pharmaceutical, biotech, and cell/gene therapy manufacturers
- Food & beverage and nutraceutical producers
- Cosmetics, personal care, and chemical industries
- Medical device and diagnostics companies
- Any site seeking to meet modern regulatory and customer audit standards
Key Benefits of Electronic Batch Record Software
Adopting electronic batch record software transforms manufacturing from a compliance risk to a proactive, quality-driven operation. Organizations gain:
- End-to-end traceability and data integrity
- Automated enforcement of approved processes and specifications
- Accelerated batch review and release cycles
- Reduction in manual errors and investigation times
- Instant, remote access for auditors and QA personnel
- Data-driven insights for continuous improvement
- Lower risk of regulatory actions and product recalls
Leading authorities—including FDA, EMA, and ISO—are clear: digital batch record solutions are the new baseline for modern manufacturing audits and inspections.
How Does Electronic Batch Record Software Work?
Electronic batch record software manages every production phase—from raw material receipt to packaging and release—by capturing instructions, process checks, operator activities, and results in real time. Data is automatically synchronized with quality management, LIMS, and ERP systems. Advanced solutions support role-based permissions, review-by-exception workflows, and digital archiving for global inspection readiness.
- Configurable workflows for any batch process
- Automated alerts for out-of-spec or missing data
- Live dashboards and reporting for QA, operations, and compliance
- Digital archiving for long-term record retention and traceability
- Support for global regulatory requirements (ICH, FDA, EMA, ISO 13485, etc.)
Example: Electronic Batch Record Software in Practice
Below is a sample page from a multi-page electronic batch record software report, illustrating live data capture, automated workflow enforcement, and digital sign-offs. This visual demonstrates the power and clarity that digital batch record solutions provide during internal reviews and regulatory inspections.
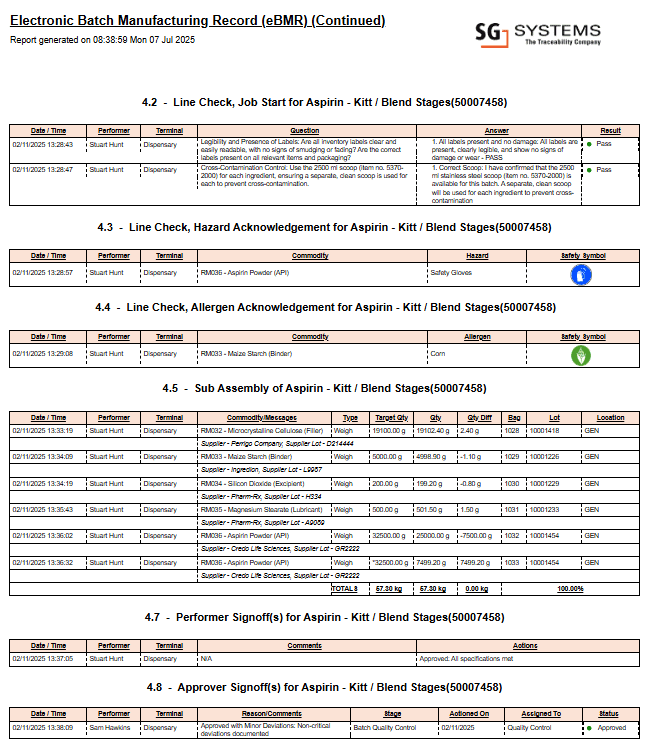
This image shows a real page from a digital batch record report, as generated by advanced electronic batch record software for regulated manufacturing.



