Digital Transformation: Electronic Batch Record Systems for Regulated Manufacturing
Electronic Batch Record Systems are fundamentally reshaping compliance, traceability, and operational performance across regulated industries—including pharmaceuticals, food, dietary supplements, and medical devices. As regulatory scrutiny and data integrity standards increase, manufacturers are rapidly replacing paper records with electronic batch record systems to achieve real-time audit readiness, secure digital traceability, and end-to-end batch visibility.
What Are Electronic Batch Record Systems?
Electronic batch record systems digitize every step of batch documentation—capturing ingredients, processes, operator actions, equipment usage, and quality checks directly from the production environment. Unlike hybrid or paper-based records, these platforms enforce process steps, require electronic signatures, and provide a continuous, audit-ready data trail for regulators and customers alike. For further reading, see FDA 21 CFR Part 11 guidance and the EMA Annex 11 guideline.
- Real-time data capture, validation, and exception management
- Automated SOP enforcement and step-by-step process guidance
- Electronic signatures with user authentication and role controls
- Live deviation, NCR, and CAPA management
- Complete audit trails and revision histories
- Integration with MES, QMS, ERP, and LIMS
Paper vs. Electronic Batch Record Systems
While paper batch records remain legal in many industries, electronic batch record systems have become the new standard. Paper is slow, error-prone, and susceptible to compliance failures. In contrast, electronic batch record systems enable live traceability, instant data retrieval, and robust regulatory defense.
| Function | Paper Record | Electronic Batch Record Systems |
|---|---|---|
| Format | Handwritten/manual | Digital, validated, compliant |
| Audit Trail | Manual, delayed, error-prone | Automated, time-stamped, audit-ready |
| Signatures | Wet ink, often incomplete | Electronic, enforced, Part 11 compliant |
| Deviation Handling | Manual, retrospective | Real-time, automated, escalated |
| Batch Review | Slow, error-prone, paper-based | Fast, digital, audit-ready |
Why Regulated Industries Choose Electronic Batch Record Systems
Compliance is non-negotiable in manufacturing regulated by GMP, ISO, FDA, or GFSI schemes. Electronic batch record systems meet the core requirements for data integrity, traceability, role-based access, and defensible audit trails. Regulatory warning letters across multiple sectors frequently cite record-keeping failures—digital batch record systems dramatically reduce these risks, automate compliance, and enable proactive quality management.
- Stronger data integrity and traceability
- Accelerated batch review and release
- Reduced compliance risk and inspection time
- Seamless integration with enterprise and quality systems
- Continuous improvement through real-time analytics
How Electronic Batch Record Systems Work
Electronic batch record systems control every stage of production. From raw material intake to final release, instructions, checks, materials, and digital signatures are recorded and validated in real time. Modern solutions provide enforceable workflows, digital sign-off, and automated transfer of data to quality, ERP, and compliance platforms. Leading platforms support requirements for food safety, pharmaceutical GMP, ISO 13485, and more.
- Automated SOPs and workflow enforcement
- Full traceability for each batch, lot, and ingredient
- Real-time integration to QMS, LIMS, and ERP
- Supports FDA, EU Annex 11, and ISO digital record requirements
Electronic batch record systems are now the baseline expectation for data integrity and regulatory compliance in modern manufacturing audits.
Example: Electronic Batch Record Systems in Action
Below is a real example excerpt from a multi-page electronic batch record system report, illustrating digital data capture, process validation, and compliance checkpoints. This image represents a single page from a complete, validated batch record system report as generated by advanced digital manufacturing solutions.
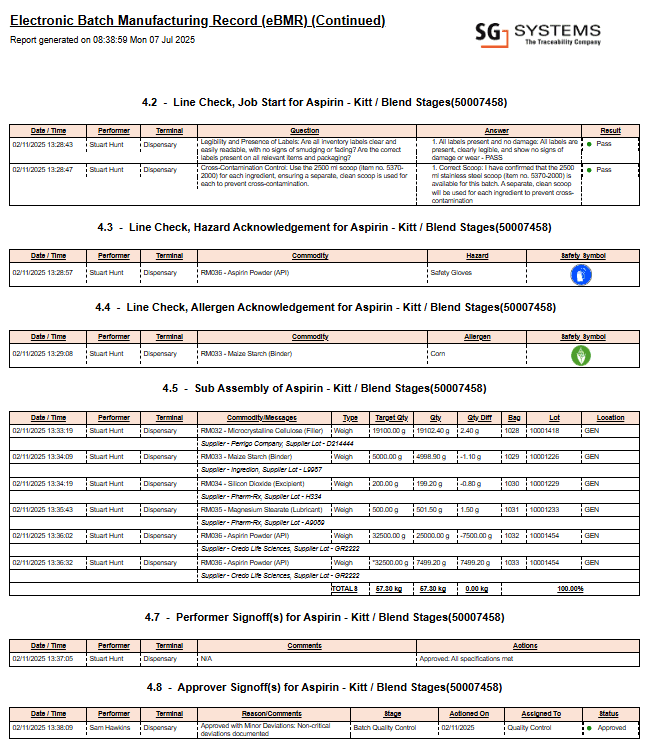
This image is an excerpt from a comprehensive multi-page electronic batch record system report generated by leading digital compliance solutions.



