LIMS Planning – Turn Labels into Controlled Lab Work
V5 from SG Systems Global makes sampling operationally unambiguous: the right label triggers the right tests on the right timeline with the right evidence. Instead of treating labels as “paperwork,” V5 treats each label as a control point that creates lab orders, locks chain-of-custody, and synchronizes with your LIMS for a closed-loop release process. The result is fewer delays, cleaner audits, and a defensible quality posture aligned to GMP, Part 11, and modern pharmaceutical quality system expectations. For manufacturers in food and supplements, the same mechanics extend to FSMA-204 traceability and GFSI frameworks like BRCGS and SQF.
“We stopped ‘printing stickers’ and started launching controlled work. Every sample label now creates a lab order with SLA tracking and audit-ready attribution.”
— QA Director, Multi-Site GMP Facility
Why Labels Must Be Control Points
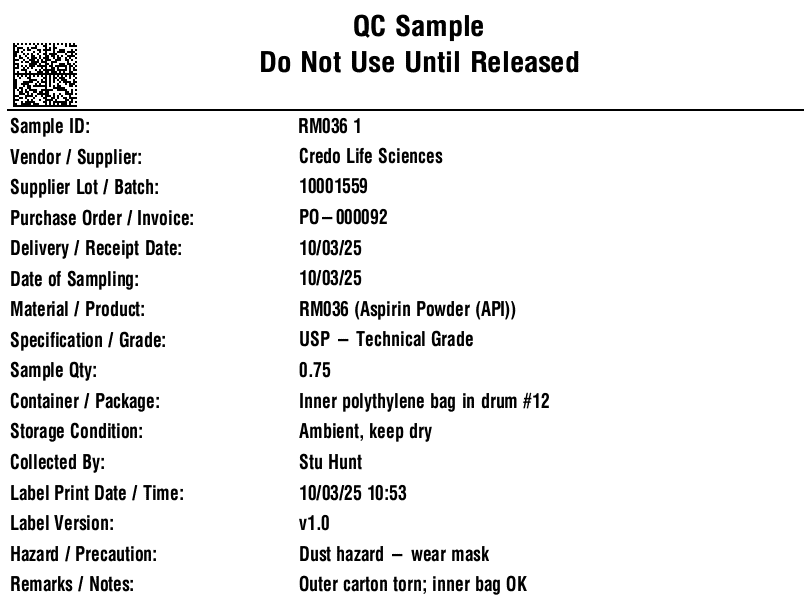
In regulated production, sampling is not a courtesy—it’s a release gate. Each lot of components must be sampled and tested with identity confirmation prior to use, and electronic systems that generate, store, and transmit these records must be trustworthy, reliable, and equivalent to paper, with secure audit trails and clear attribution. V5 operationalizes these requirements by binding master data, label templates, sampling plans, and user permissions into a single transaction: no compliant label, no material move, no release.
The V5 Sample Object – A Practical Data Model
V5 standardizes a “Sample” object so that lab work is deterministic and traceable across raw materials (RM), work-in-process (WIP), finished goods (FG), retains, and stability pulls. The core fields include:
- Identifiers: SampleID (1D/2D barcode), MaterialCode, BatchID, LotID, optional ContainerID for split samples/retains.
- Context: SampleType (RM/WIP/FG/Retain/Stability), manufacturing step, storage condition (2–8 °C/−20 °C/ambient), and location.
- Test Plan: Named panel (e.g., RM-Identity-Assay-Micro), priority/SLA, method references, and special instructions.
- Governance: TemplateVersion, OperatorID(s), timestamp, reason-for-change, e-signatures where required.
- Linkages: Supplier details, inbound documentation, MMR/EBR pointers, deviation/CAPA references.
These fields are governed under role-based access and change control. Electronic record integrity aligns to Part 11 expectations (unique credentials, tamper-evident audit trails, validated behavior), and quality-system governance follows risk-based, lifecycle-aware best practices.
From Label to LIMS – Scheduling That Runs Itself
In V5, printing a sample label is a transaction that creates a lab order and populates the LIMS schedule. The data packet includes sample type, batch/lot, storage condition, test panel, and due-by SLA. LIMS executes; results and dispositions write back automatically to update holds, batch records, dashboards, and release signatures. Integration patterns can be file-based (CSV/XML/JSON to validated directories), API-based (REST/gRPC), or brokered via middleware—each validated for integrity, reliability, and auditability.
- Priorities & SLAs: Risk-weighted queues for new suppliers, high-criticality materials, or lots with OOS history.
- Campaigning: Group related tests to minimize bench context switching while preserving traceability.
- Time-Phased Tasks: Retains and stability at T=0/1/3/6/9/12 (or your scheme), with escalations for missed pulls.
- Closed-Loop Write-Back: Holds clear only when result criteria are met; signatures and timestamps remain compliant.
If you run testing in-house, laboratory competence and impartiality expectations are supported by preserving method IDs, instrument references, and calibration traceability in the record so investigations move quickly.
Operator Experience – RM, WIP, FG (One Pattern, Fewer Errors)
- Raw Materials (RM): Scan inbound receipt; V5 checks approved supplier status and inbound documentation, then prints an RM sample label. Inventory remains quarantined until identity (and other required tests) pass.
- WIP: Trigger sampling at post-weigh, pre-blend, pre-fill, or other risk-based steps; labels inherit batch/step context for SPC and trending.
- FG: Final sampling fires release tests plus retain and stability lots; passing results close holds and complete the EBR with review-by-exception.
Because the pattern is consistent, training time drops while data integrity rises. Each print captures who/when/where/why, and template versions prevent “creative” labels from bypassing governance.
Data Integrity by Design
V5 enforces ALCOA/ALCOA+: attributable scans, readable and versioned templates, contemporaneous capture at point-of-work, storage of original records/true copies, and accuracy controls (controlled vocabularies, method IDs, range/format checks). Training and permissions ensure only qualified users can execute or approve critical steps, with tamper-evident audit trails backing every action.
“Our biggest gain wasn’t speed—it was confidence. Every transfer, split, and storage move is attributed and time-stamped, so root cause isn’t a guessing game.”
— Laboratory Manager, Solid Dose Facility
Risk-Based Sampling That Matches PQS
More testing where uncertainty is high (e.g., new API suppliers, complex matrices); reduced sampling where capability is proven and supplier performance is stable—subject to procedures and validation evidence. V5 codifies these decision rules in the sampling plan and binds the rationale to change control so auditors see not just the outcome but the reasoning.
Failure Modes V5 Removes
- Mislabeling: Version-controlled templates, locked fields, and scanner-enforced selection prevent operator “creativity.”
- Missed Timepoints: Calendarized retains/stability with escalations stop drift and protect shelf-life studies.
- Custody Gaps: Every move is a scan event with location and user attribution; split containers retain parent-child lineage.
- Re-entry Errors: Direct LIMS write-back eliminates copy/paste into EBRs; approvals and holds are updated automatically.
Metrics That Matter
- ≥ 99.9% right-first-time label accuracy (template lock + scan enforcement)
- ≥ 95% lab SLA adherence (priority-aware scheduling + escalations)
- ≥ 90% review-by-exception release coverage (results flow straight into EBR)
- 30–50% rework reduction (no manual transcription of lab data)
Industry Contexts (Pharma, Supplements, Food)
Pharmaceuticals: Representative sampling and identity confirmation of each component lot before use are non-negotiable; V5 encodes these rules in receiving and sampling so materials cannot progress until released by the quality unit.
Dietary Supplements: Identity is a baseline, with potency and contaminant testing enforced by procedure; V5 preserves supplier qualification and COA verification logic with audit trails.
Food & Beverage: FSMA 204 requires additional traceability records for items on the Food Traceability List (FTL), with Key Data Elements (KDEs) captured at Critical Tracking Events (CTEs). V5 labels and transactions carry traceability lot codes and KDEs from receiving through transformation and shipping, and can retrieve records within regulatory response windows.
Certification Frameworks: BRCGS and SQF emphasize forwards-and-backwards traceability, recall readiness, and rapid record retrieval. V5’s chain-of-custody and recall reporting align with both, reducing audit friction.
Role Clarity – Production, QA, Lab
- Production: Initiates sampling at control points; cannot progress when gates (labels, holds) are unmet.
- QA: Owns label template governance, sampling plans, change control, and release decisions.
- Lab: Executes tests; posts results and investigations (OOS/atypical) with method/instrument traceability.
Exception Handling and Investigations
When results are delayed or out-of-spec, V5 routes the batch into a controlled state with deviation/CAPA records. Because every event—from label print to storage move to analyst sign-off—is time-stamped and attributable, investigators get sequence certainty, not inference. Audit trails and permissioning make expectations provable.
Food Traceability Specifics – KDEs and CTEs
If you process items on the FTL, you must capture specific KDEs at each CTE and be able to retrieve them rapidly during an event. V5 binds the KDEs to transactions (receiving, transformation, shipping), applies uniform traceability lot codes, and generates recall/market withdrawal reports for swift response and audit readiness.
Implementation Plan (Fast, Validated, Defensible)
- Pilot a High-Volume RM: Lock label template; enforce identity testing; validate “no label, no move” at receiving.
- Map LIMS Integration: Choose file/API/middleware; conduct closed-loop testing (order → result → hold clear → EBR/signature); capture validation evidence.
- Add WIP/FG Checkpoints: Include retains and stability pulls with time-phased tasks and SLA escalations.
- Governance & PQS: Put template edits and risk assessments under change control; ensure lab competence or vendor qualification.
- Scale Across Sites: Standardize sample types, plan codes, method IDs; monitor KPIs (label RFT, SLA hit rate, review-by-exception coverage) and tighten thresholds as capability improves.
Explore Related Controls in V5
- Electronic Batch Record (EBR) – results, holds, and sign-offs flow directly from LIMS into release documentation.
- Master Manufacturing Record (MMR) – sampling points and methods governed alongside manufacturing steps.
- Quality Management System (QMS) – deviations, CAPAs, and change control tied to sampling and results.
- V5 Connect (API) – validated integrations for LIMS/file drops, ERP, and data lakes.
Bottom line: a sample label should cause the correct lab work, on time, every time. Treating the label as a control point—governed, attributed, and integrated—shrinks release cycles, elevates data integrity, and turns audits into demonstrations rather than debates.