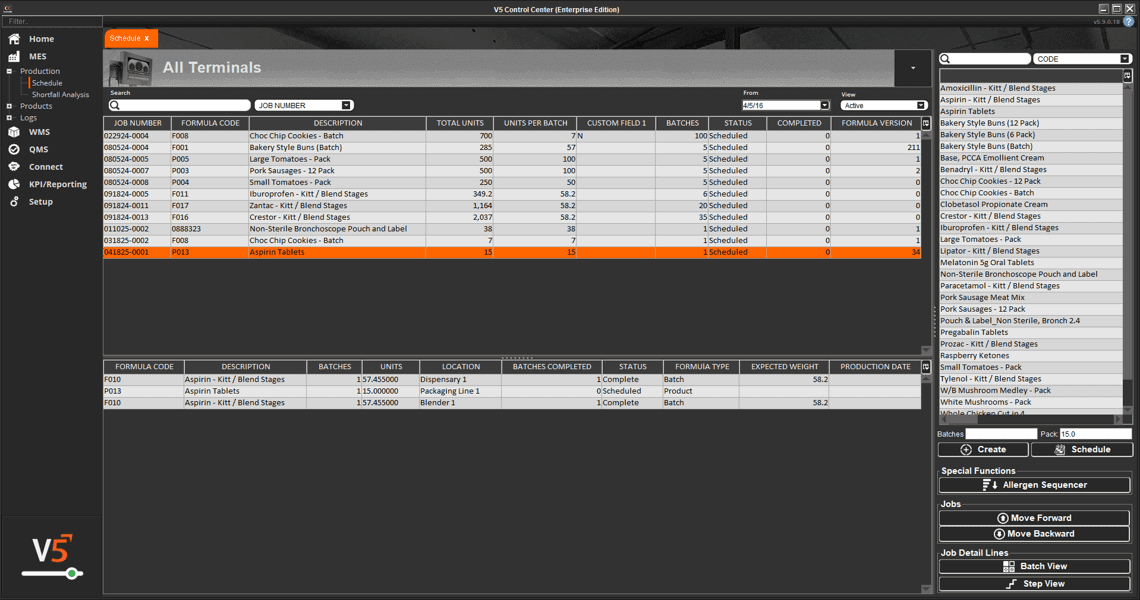
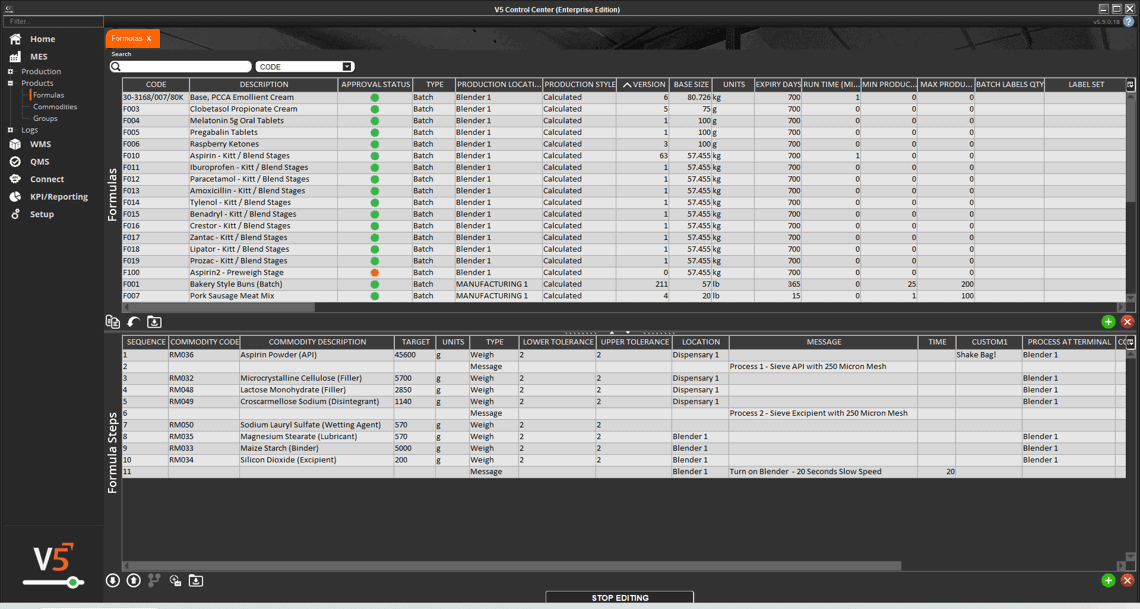
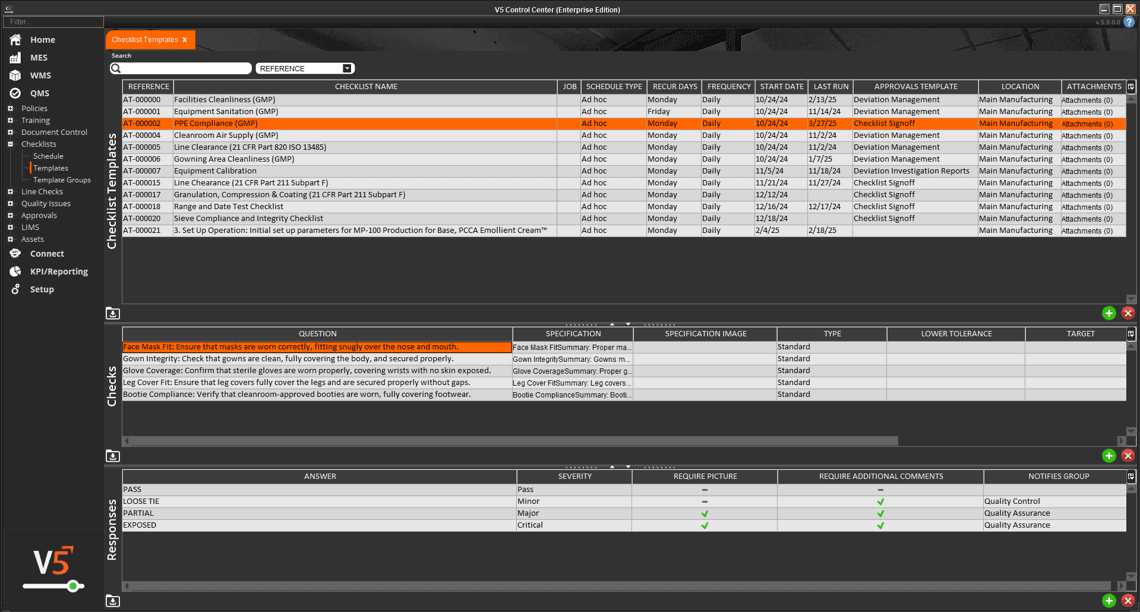
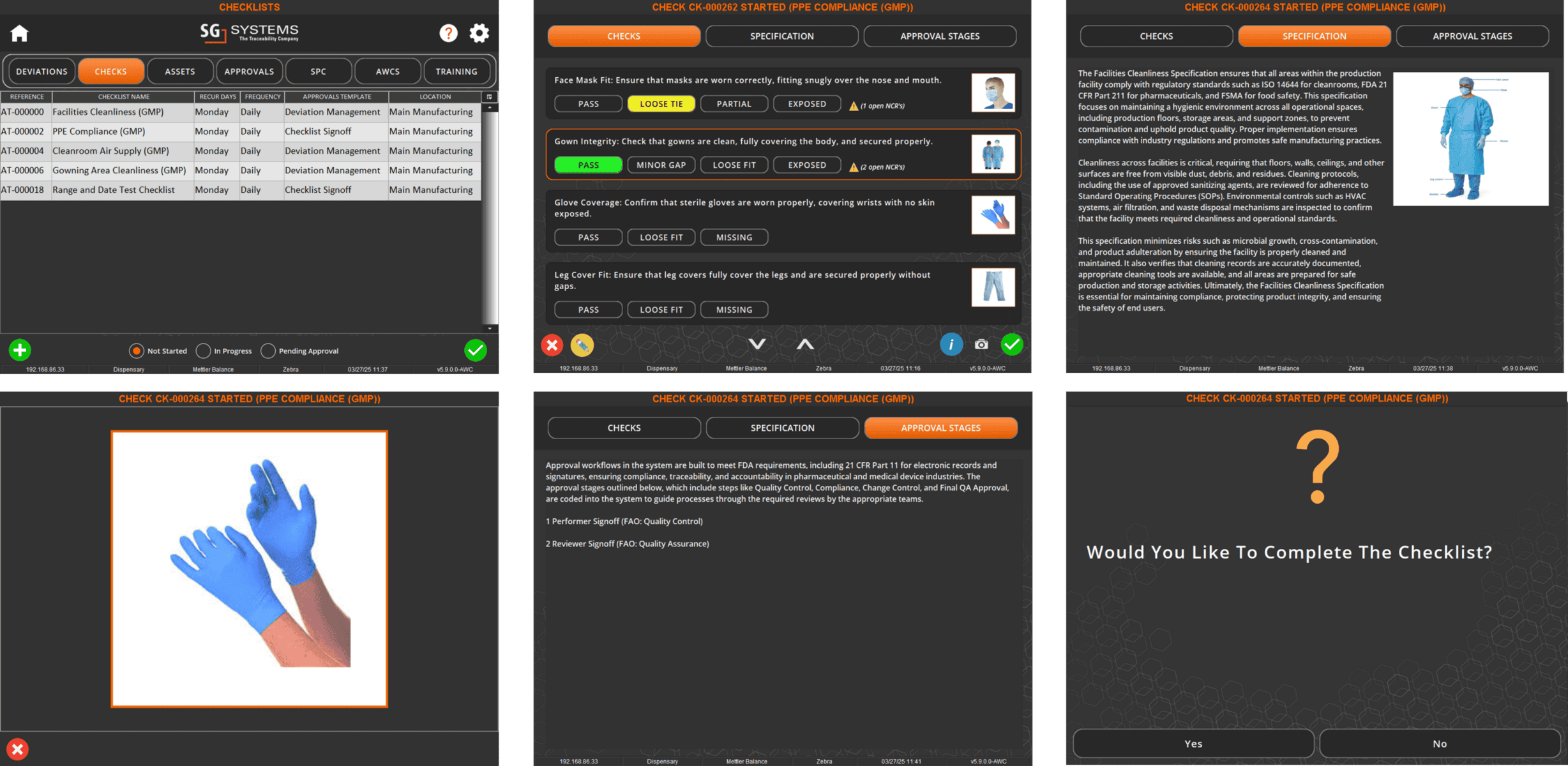
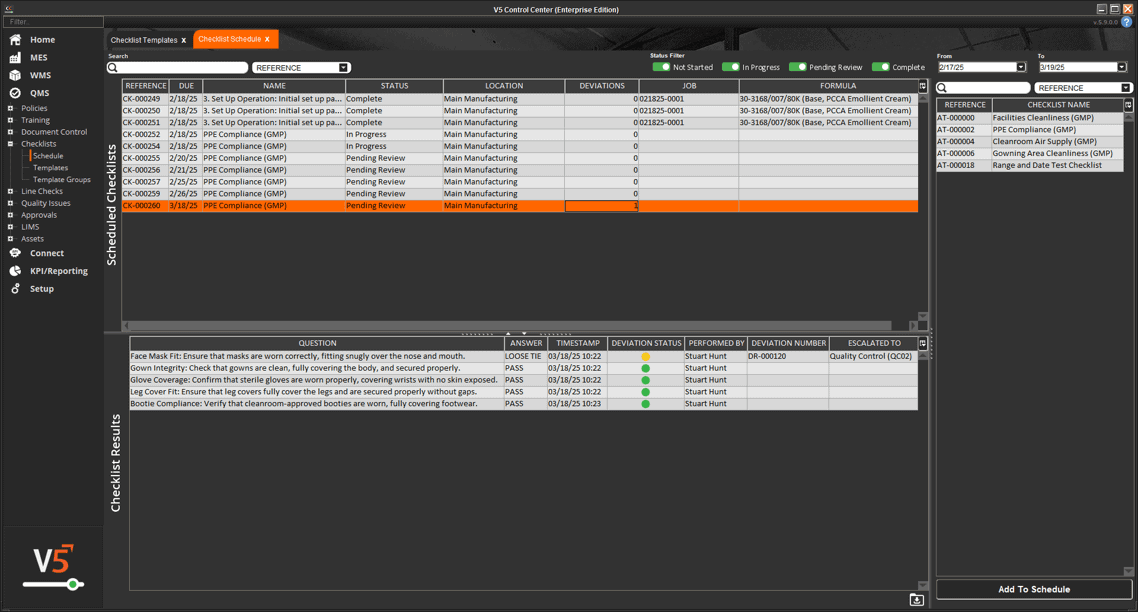
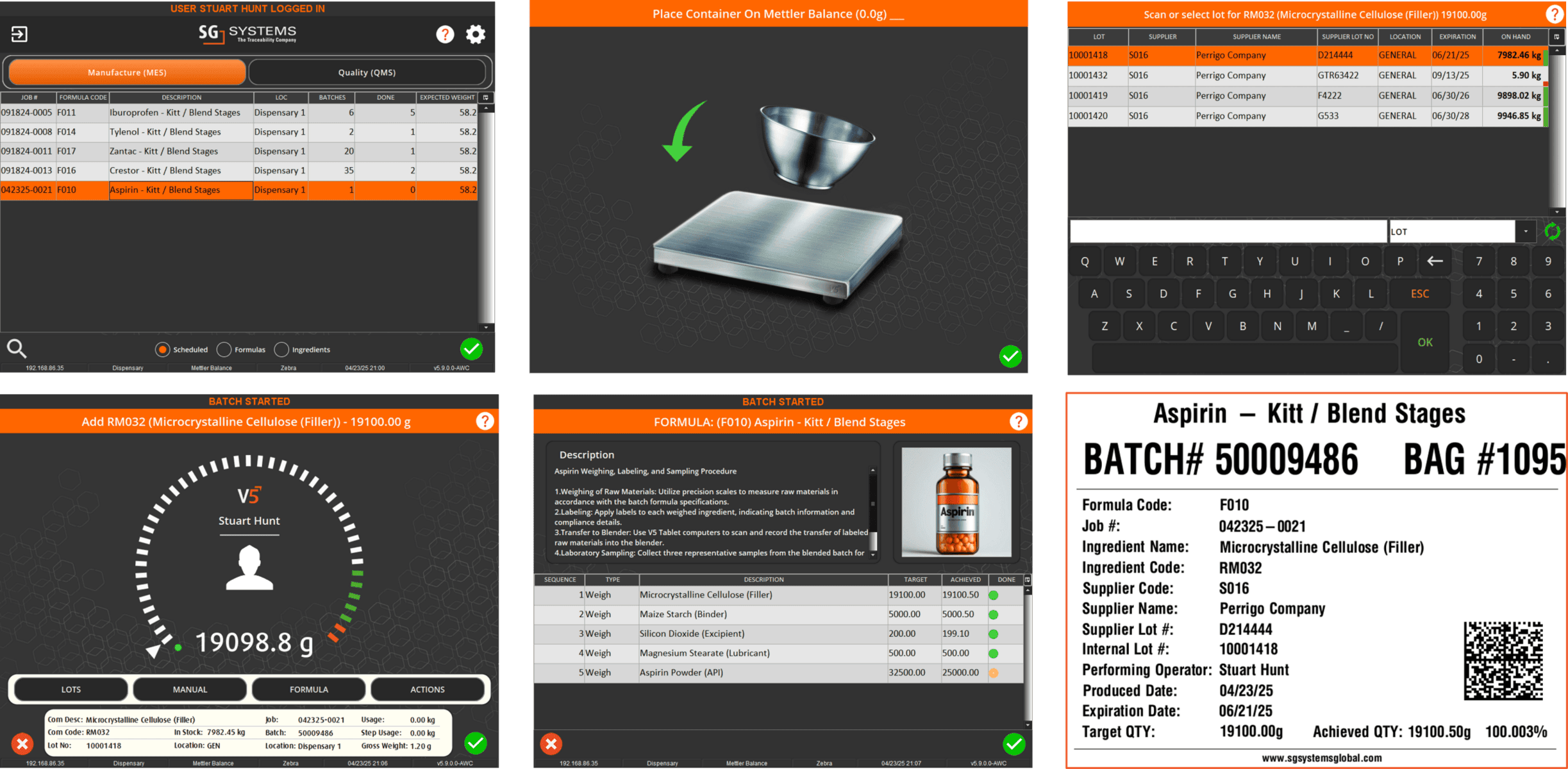
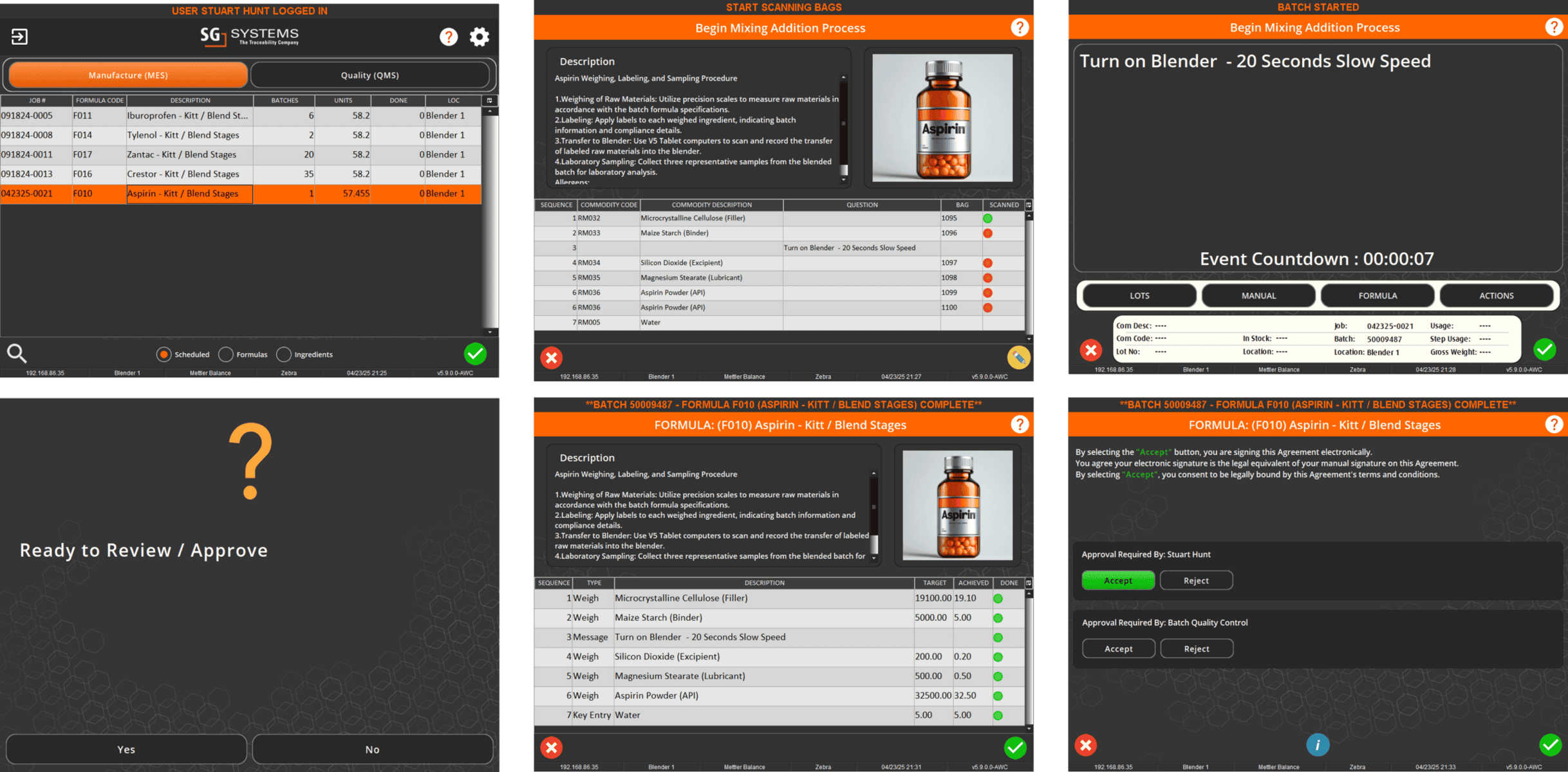
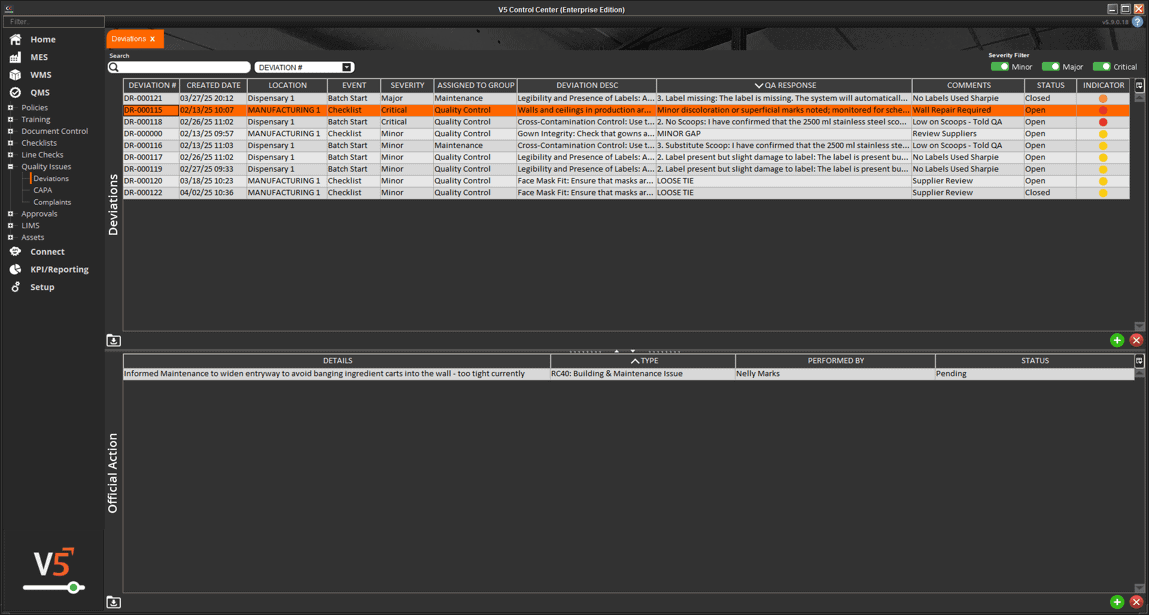
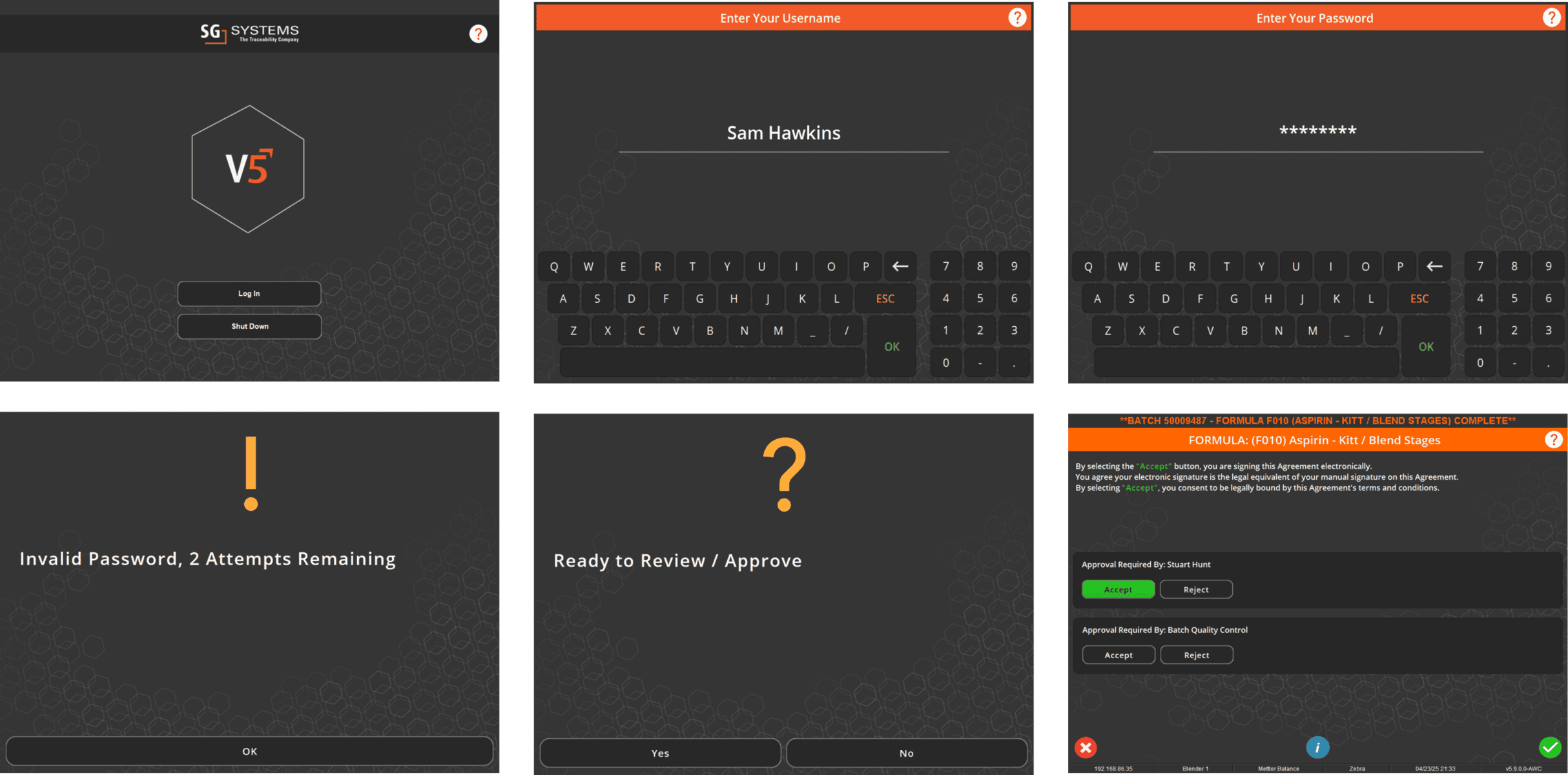
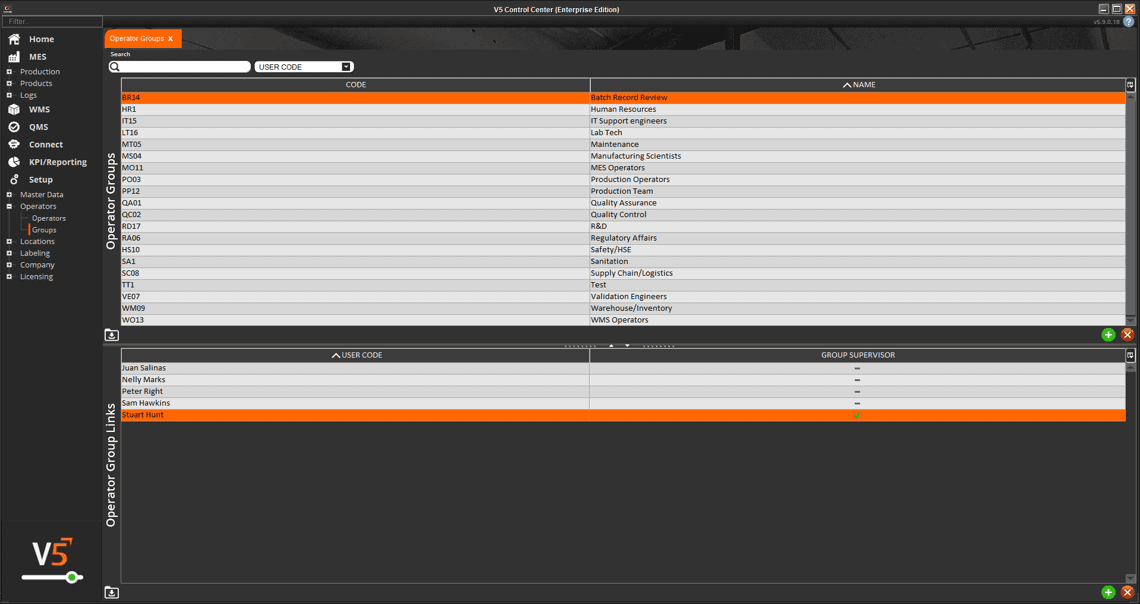
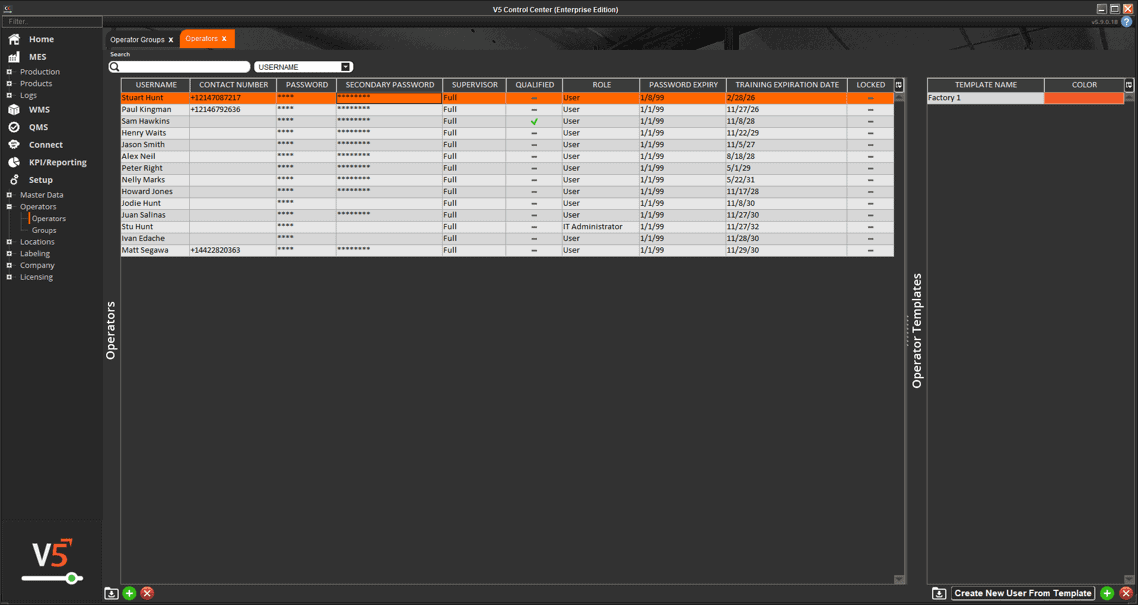
In V5, assets aren’t just listed—they’re managed, monitored, and tied directly into production execution. Every scale, mixer, filler, and cleanroom device is tracked with usage logs, calibration history, and maintenance schedules. Operators can only use calibrated equipment, spare parts are linked to inventory, and preventive maintenance tasks are automatically scheduled before downtime occurs.
The screen below shows how V5 integrates equipment data with production workflows, ensuring each asset is validated and ready before it’s used. From calibration sign-offs to maintenance status, nothing is left to chance.
Key Benefits:
Centralized register of all assets, equipment, and tooling
Automated calibration scheduling with enforced lockouts on overdue assets
Preventive maintenance plans tied to usage hours, cycles, or time intervals
Spare parts ordering and consumption tracking linked to WMS inventory
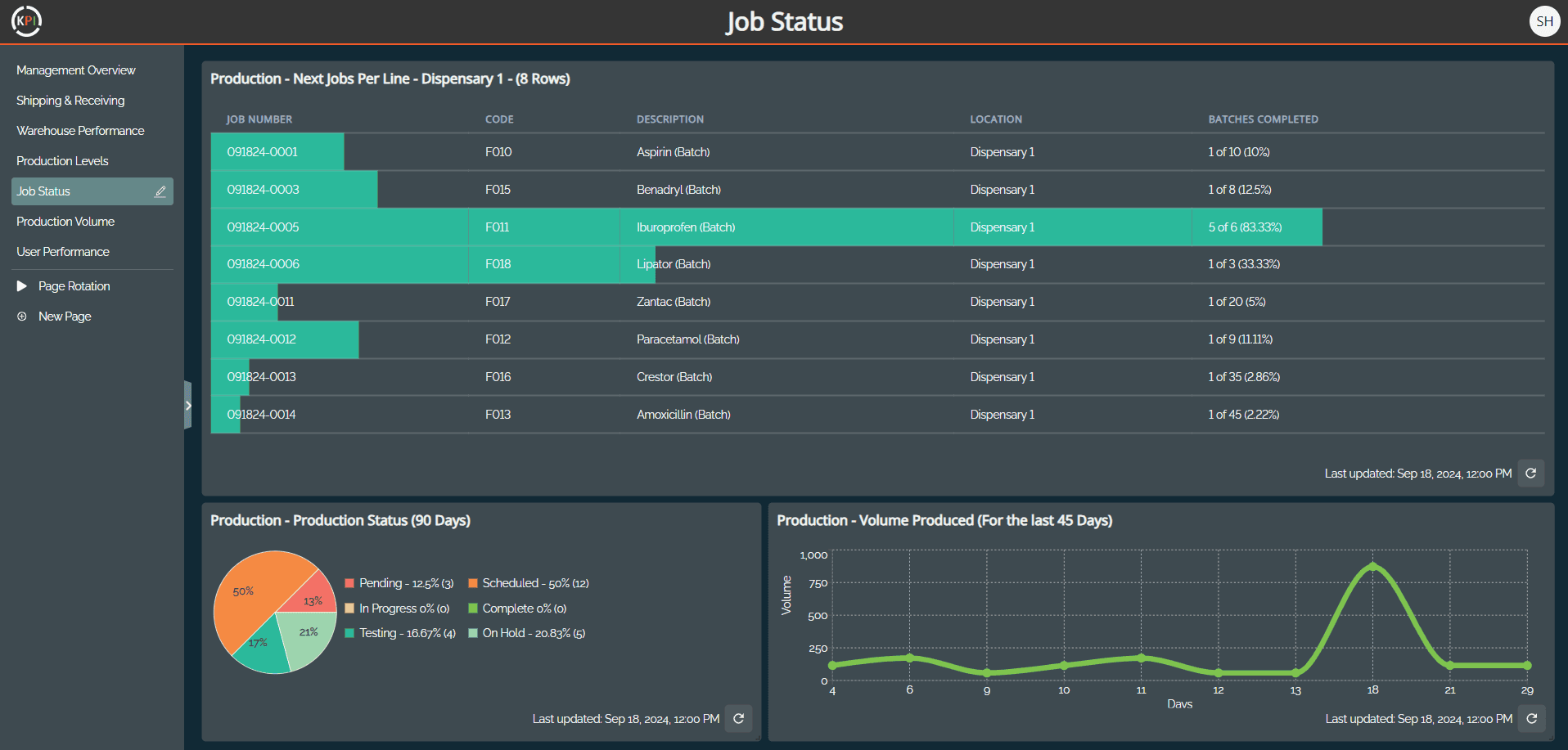
Real-time visibility into equipment status (available, under maintenance, out of calibration)
Full traceability of asset history linked to batch records for compliance audits
V5 makes assets accountable.
Instead of reacting to equipment issues or missing calibration logs, you’ll have a live system that enforces readiness, prevents downtime, and proves compliance. With V5, assets aren’t just tracked—they’re controlled.