The Importance of Weighing Traceability in Production
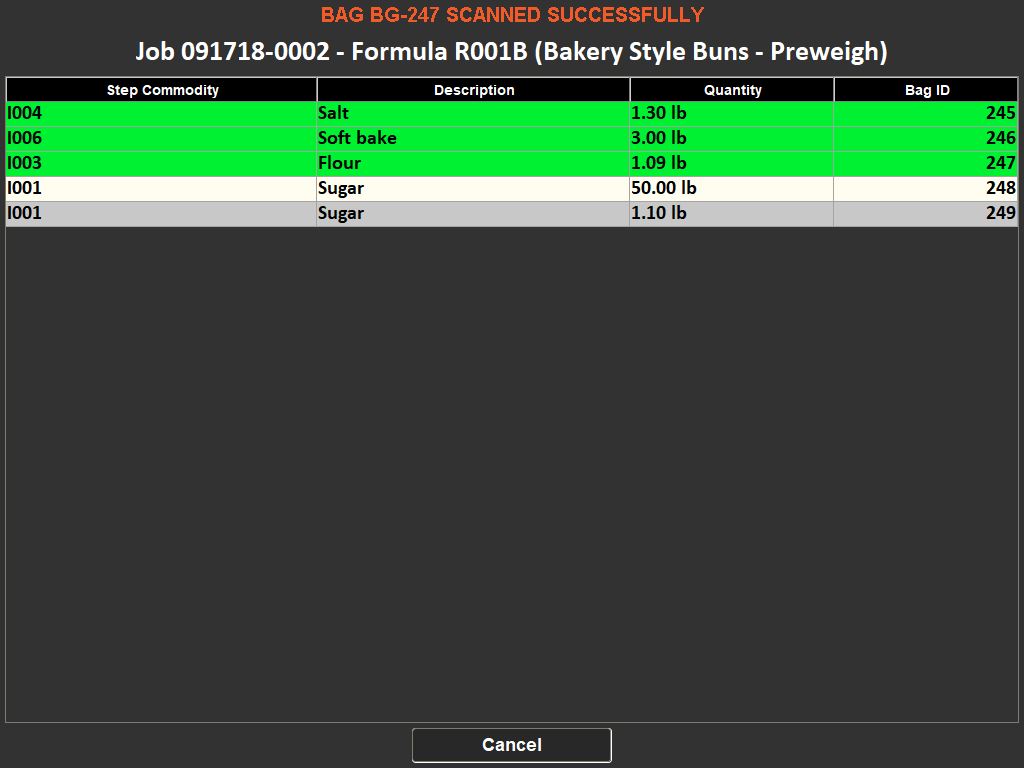
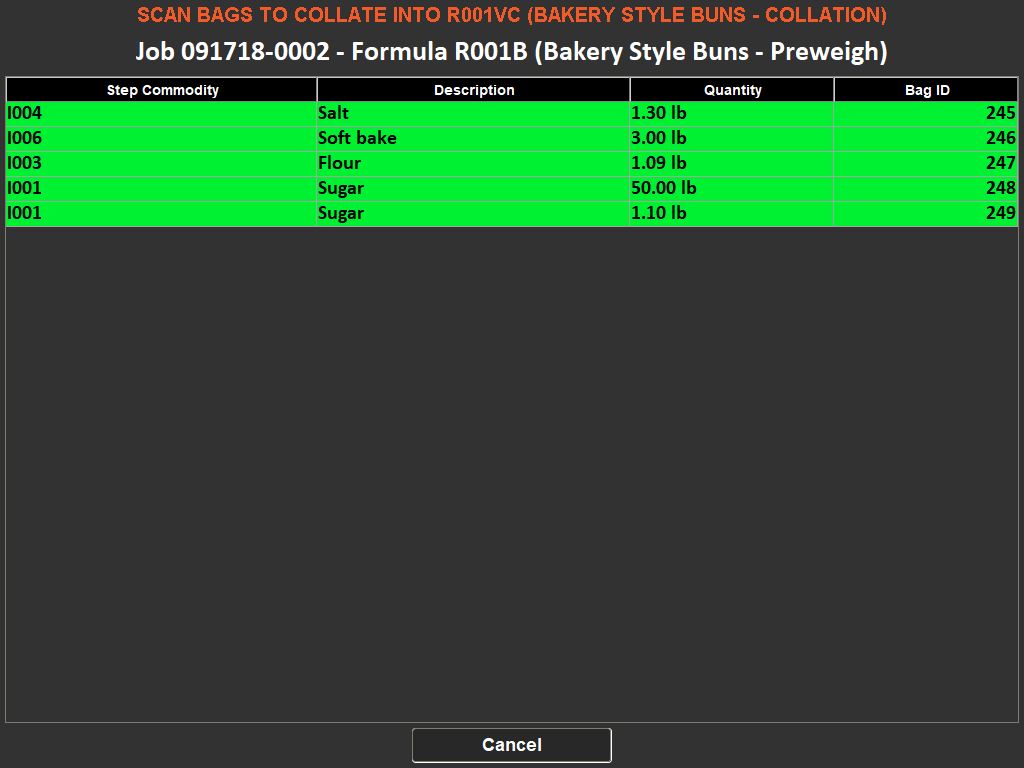
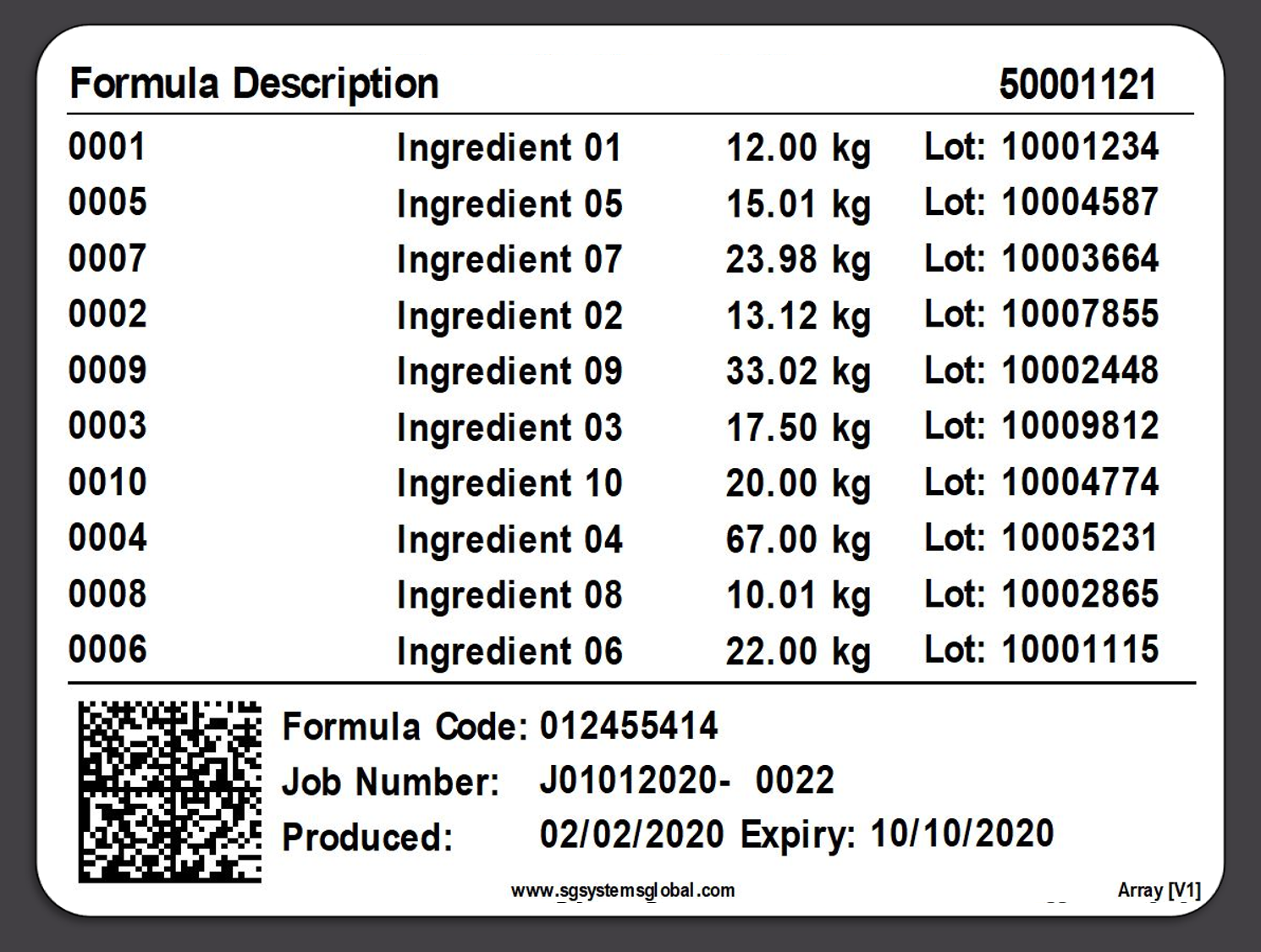
Weighing traceability in production is an essential component in maintaining the integrity and quality of the manufacturing process. It involves the accurate measurement and tracking of ingredients or components, ensuring that each batch produced adheres to the specified formula with precise quantities. This level of accuracy is crucial in industries where the slightest deviation can impact the quality, safety, or efficacy of the final product, such as in pharmaceuticals, food production, and chemicals.
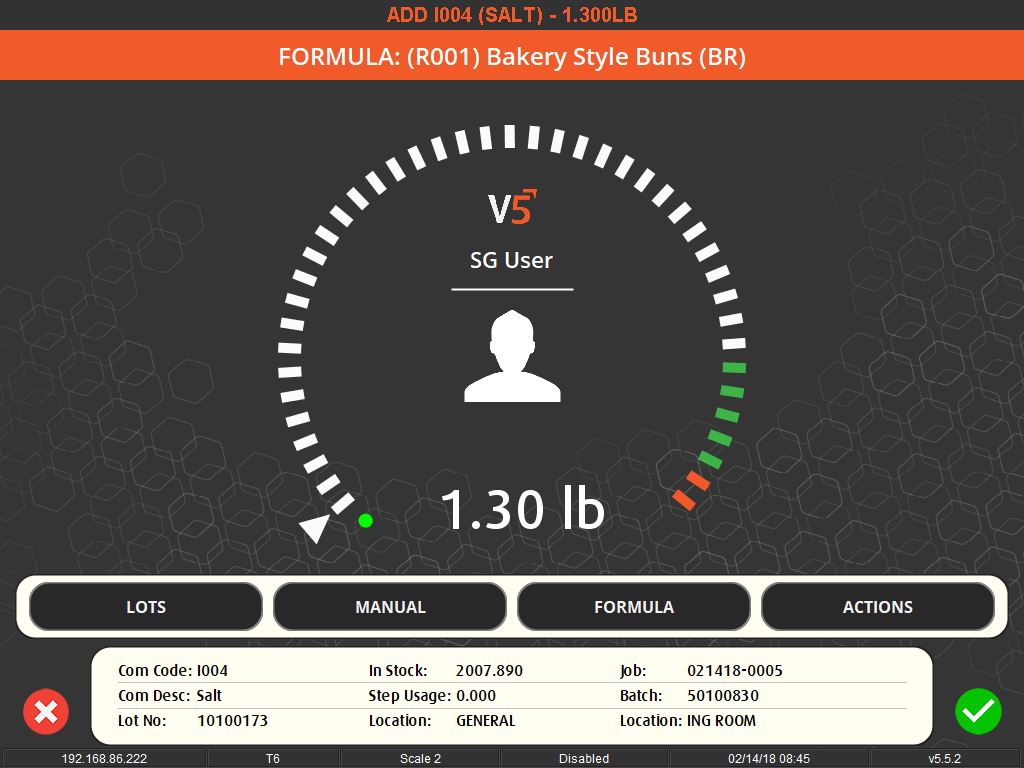
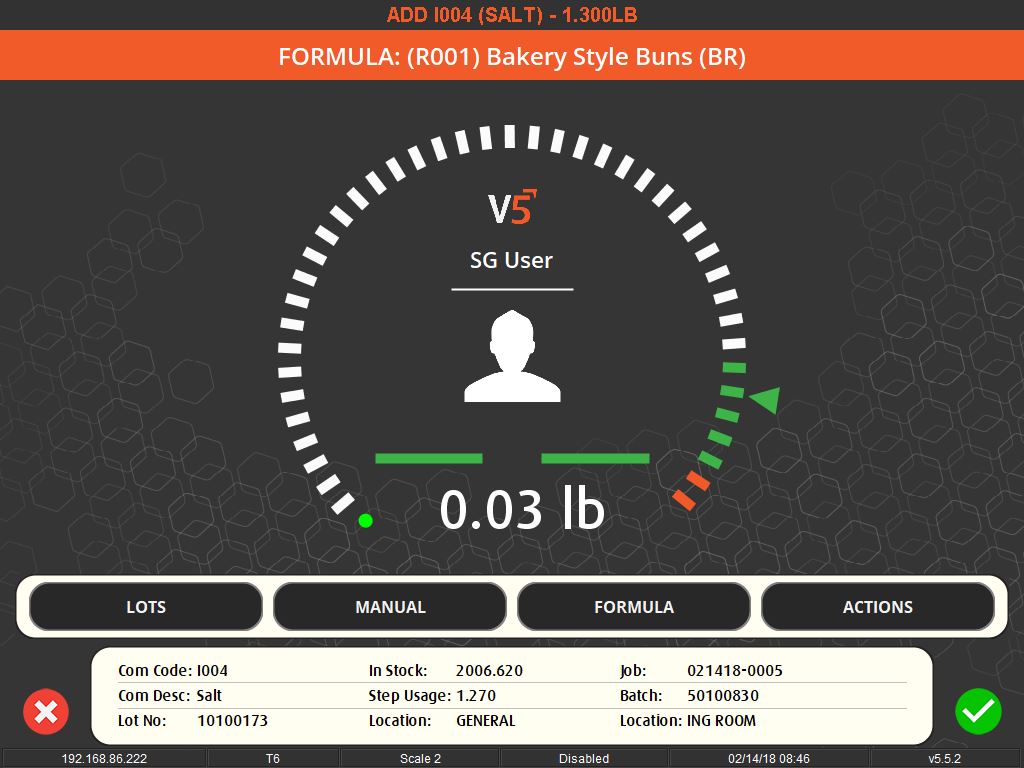
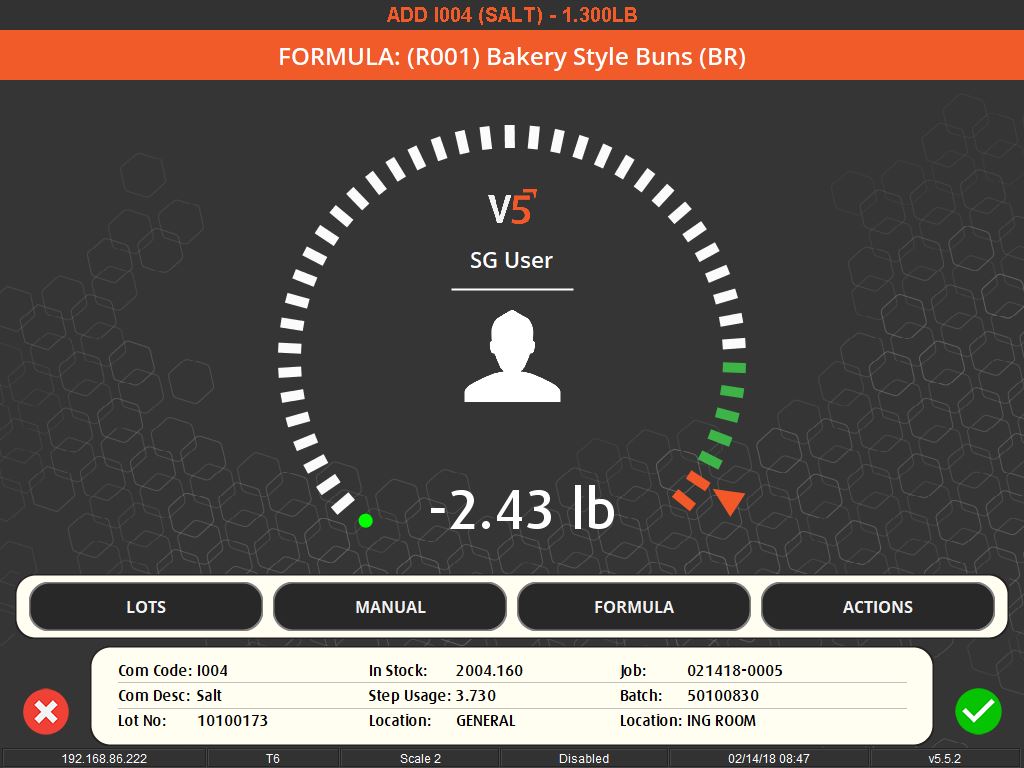
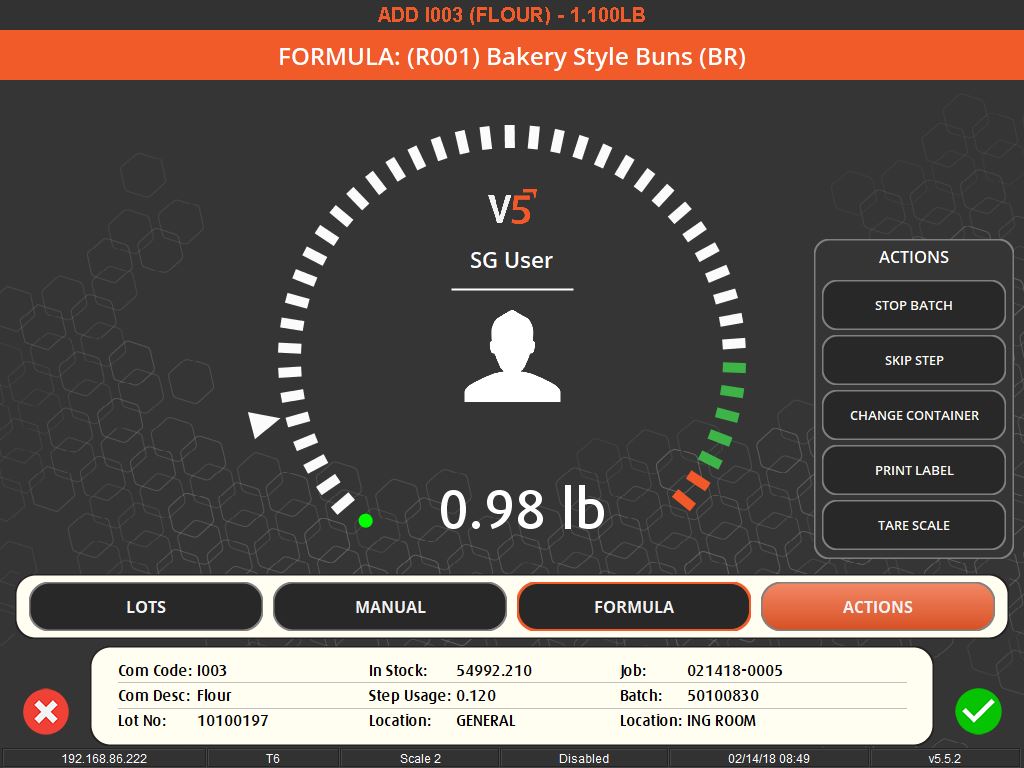
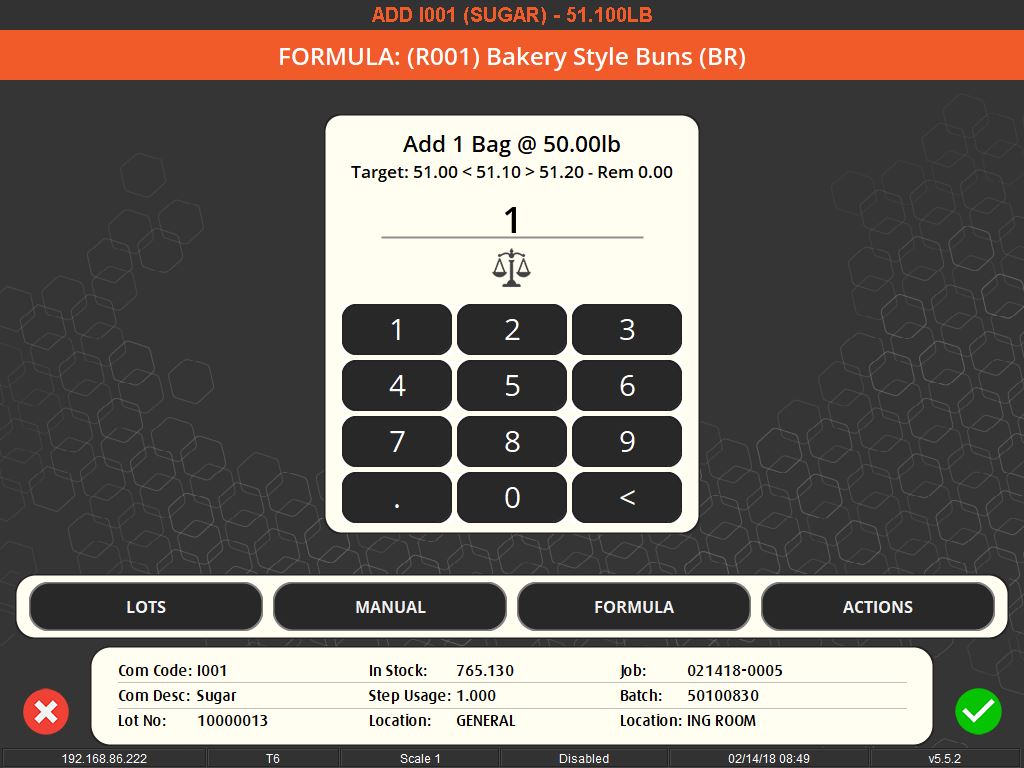
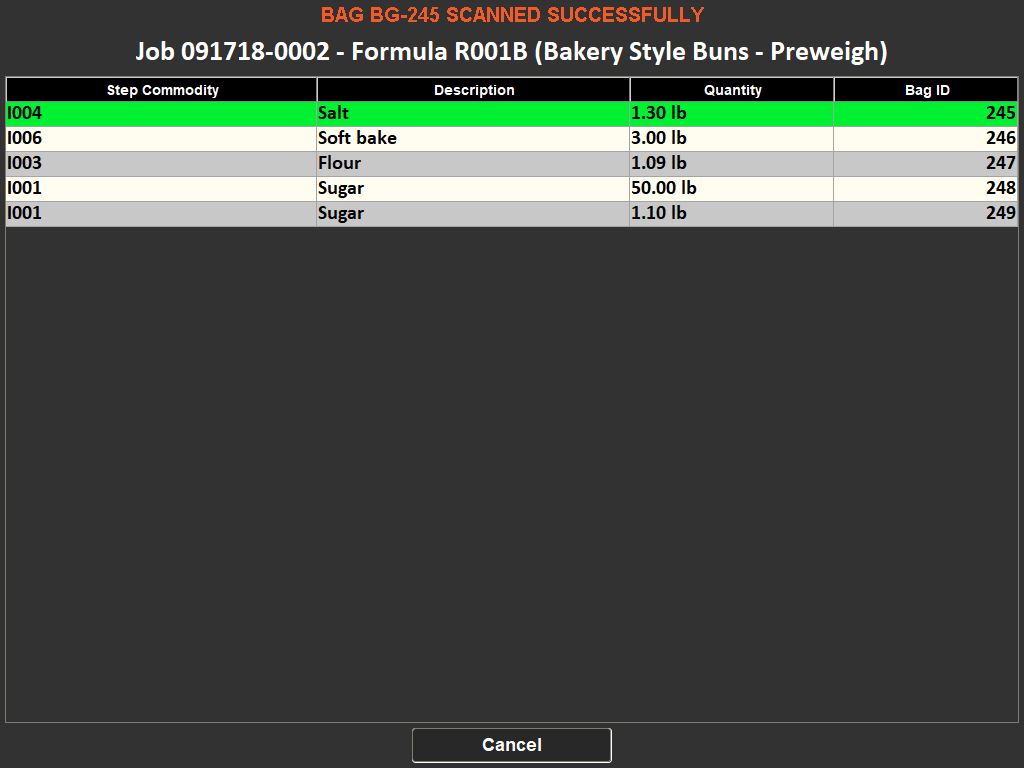
The importance of weighing traceability lies in its ability to minimize errors and waste. By accurately measuring ingredients, manufacturers can avoid costly mistakes such as overusing expensive materials or producing out-of-specification batches, which can lead to waste or rework. This accuracy is enforced through systems that set unique tolerances for each ingredient, preventing the completion of a recipe until all components are measured correctly.
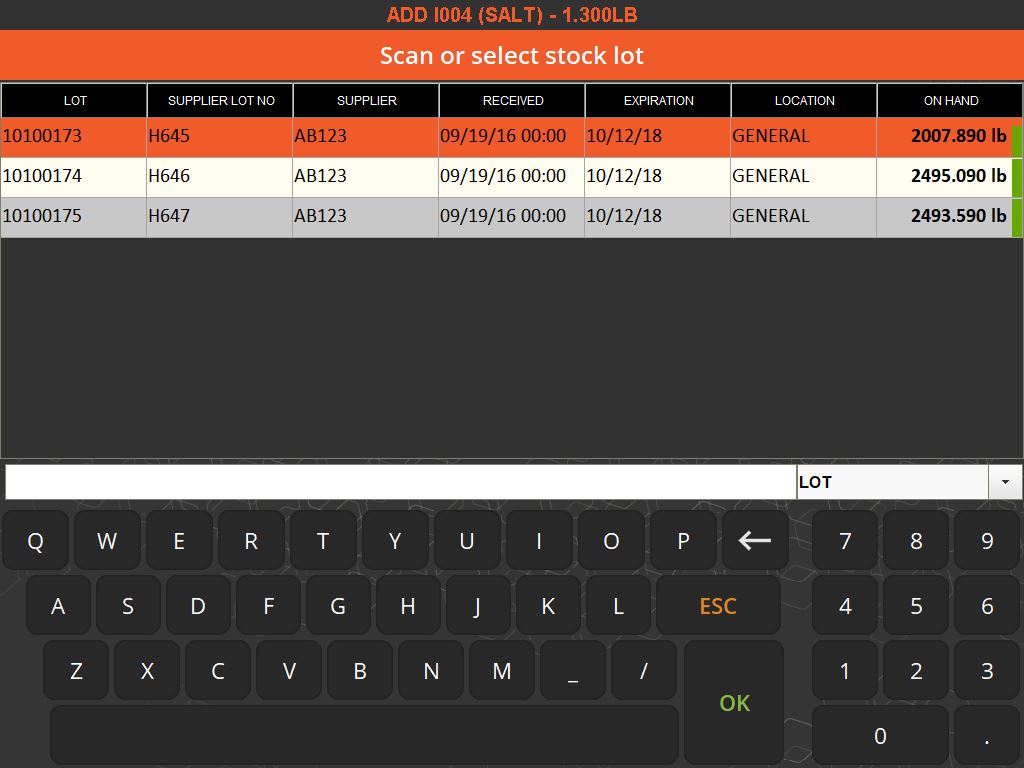
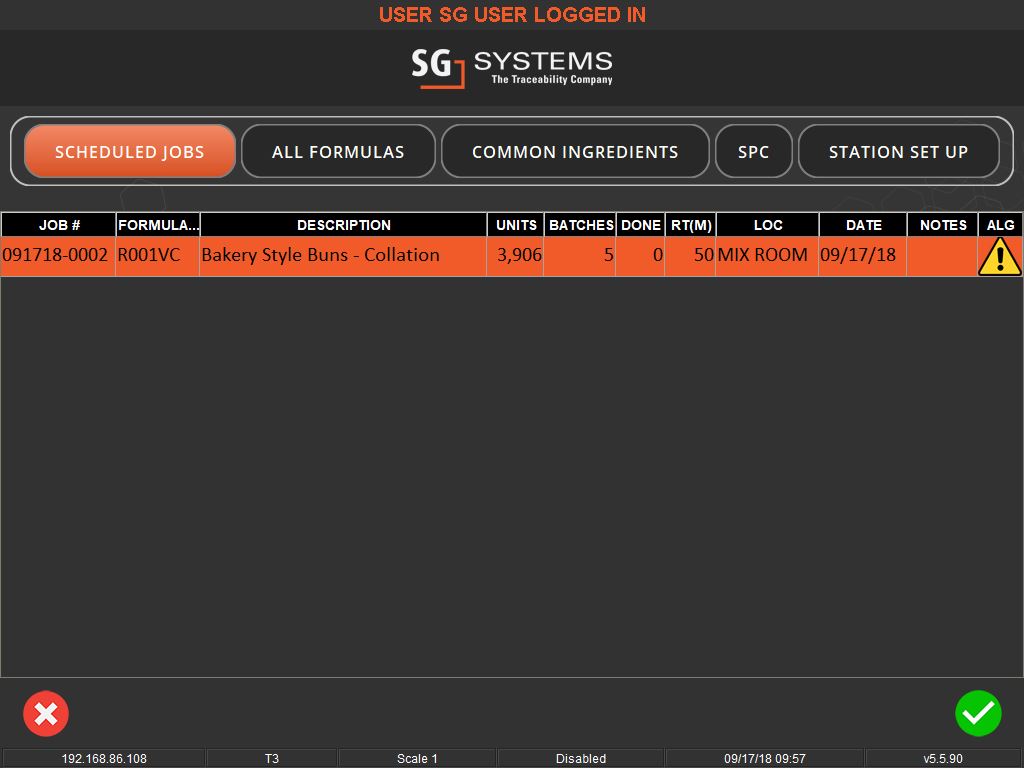
Integration with Enterprise Resource Planning (ERP) systems enhances the efficiency of weighing traceability. This integration allows for real-time monitoring of inventory usage, aiding in better resource planning and reducing the need for extensive physical inventory checks. It also streamlines the documentation process, replacing manual record-keeping with digital tracking, which is not only more efficient but also reduces the risk of human error.
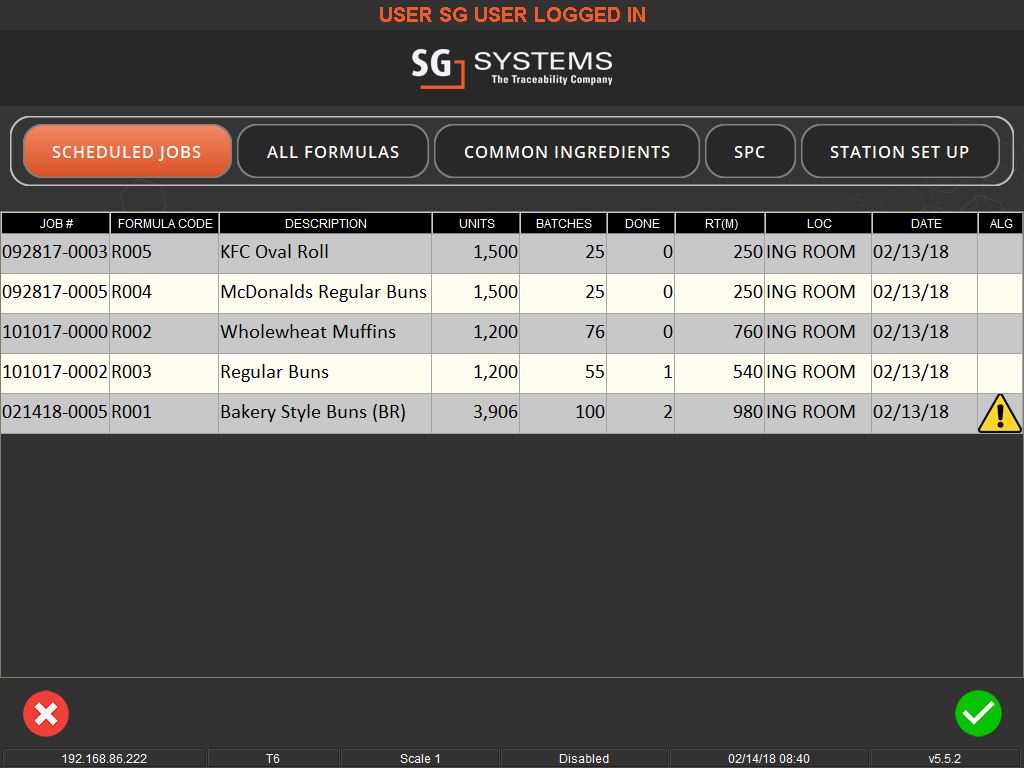
User-friendly interfaces and flexible systems are key to effective weighing traceability. These systems allow for easy scheduling of production jobs and can accommodate various methods of ingredient addition, whether it’s through key entry, counting, or bulk control. They also offer different levels of access and control based on operator logins, ensuring that each step of the process is monitored and recorded.
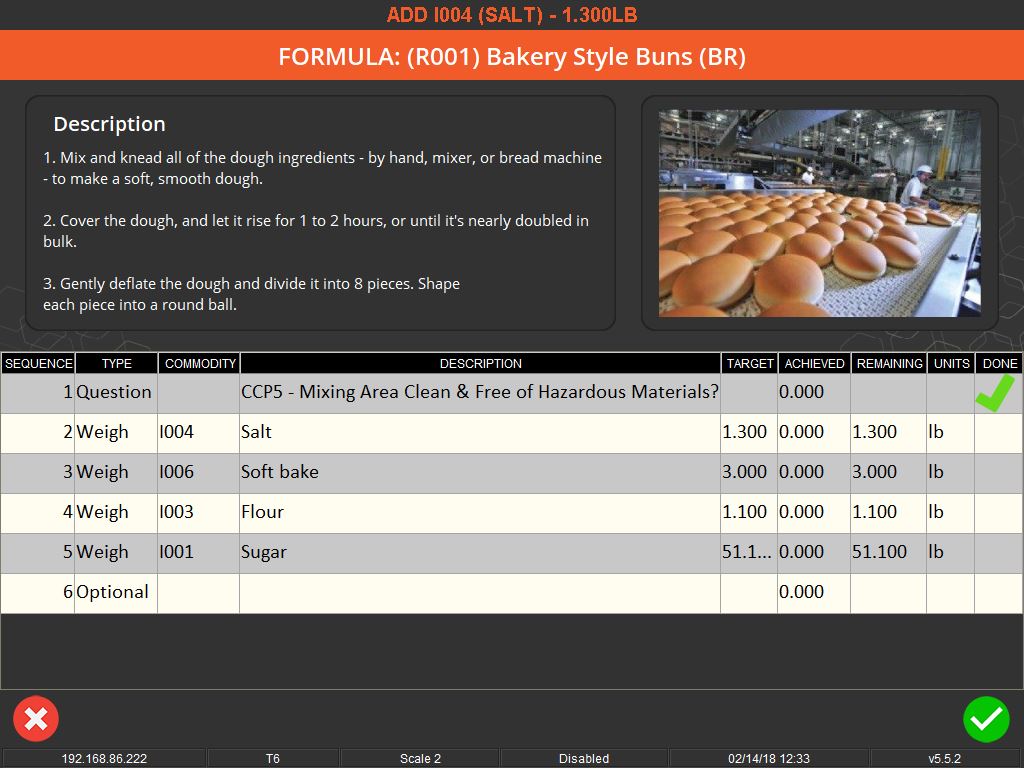
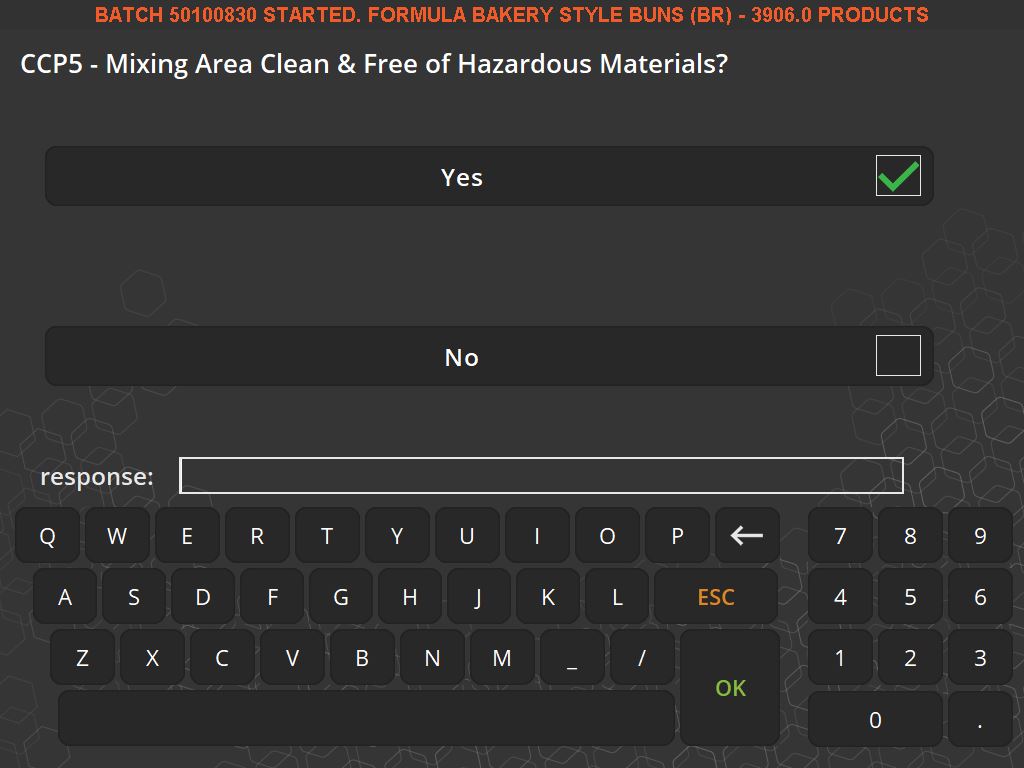
In addition to ensuring accuracy and efficiency, weighing traceability plays a crucial role in compliance and quality assurance. Many industries are subject to strict regulatory standards, and traceability systems help ensure that these standards are met. They can display important information such as allergen warnings, safety instructions, and compliance with Hazard Analysis Critical Control Point (HACCP) and Good Manufacturing Practices (GMP).
Moreover, these systems contribute to a safer working environment. By ensuring that only the correct, serialized ingredients are added to a batch, they prevent the risk of contamination or incorrect formulations. They also support a paperless quality assurance environment, recording responses to batch-specific quality questions and facilitating the tracking of quality standards.
Overall, weighing traceability is a vital aspect of modern manufacturing, providing a foundation for accurate, efficient, and compliant production processes. Its role in ensuring product quality, reducing waste, and adhering to regulatory requirements cannot be overstated, making it an indispensable tool in the arsenal of modern manufacturing.