
News

Backward Traceability Report
SG Systems is proud to announce the Backward Traceability Report, enabling 100% electronic traceback with complete confidence, now including submix components. As a major food manufacturer, handling a fussy auditor is one thing, but taking a recall from a major customer is another.
It can be a deal-breaker if you cannot produce accurate traceability within hours. The longer the recall takes, the less confidence your customer will have in your product and recall systems. Four hours has recently become the ‘time to beat’ but this can be a daunting task for anybody relying on a manual paper-based system (more if you have submix components within your manufacturing process). If you have ERP it shouldn’t be too tricky. Still, it’s reliant on manually keystroking data into the system, which is problematic and comes at a high labor cost, and delivers no return on investment.
Customer-led recalls, often termed ‘Backwards Traceability’ can put companies into huge loss-making propositions. Producing and distributing fresh products – ‘pull everything’ may be a solution. However, pulling everything from the shelves is costly, time-consuming for the distributor and expensive for everybody.
Companies producing frozen products cannot simply ‘pull everything.’ Withdrawing six months of production because a recall cannot be completed quickly and surgically can be catastrophic and sometimes lead to the end of the business.
Backward Traceability Report
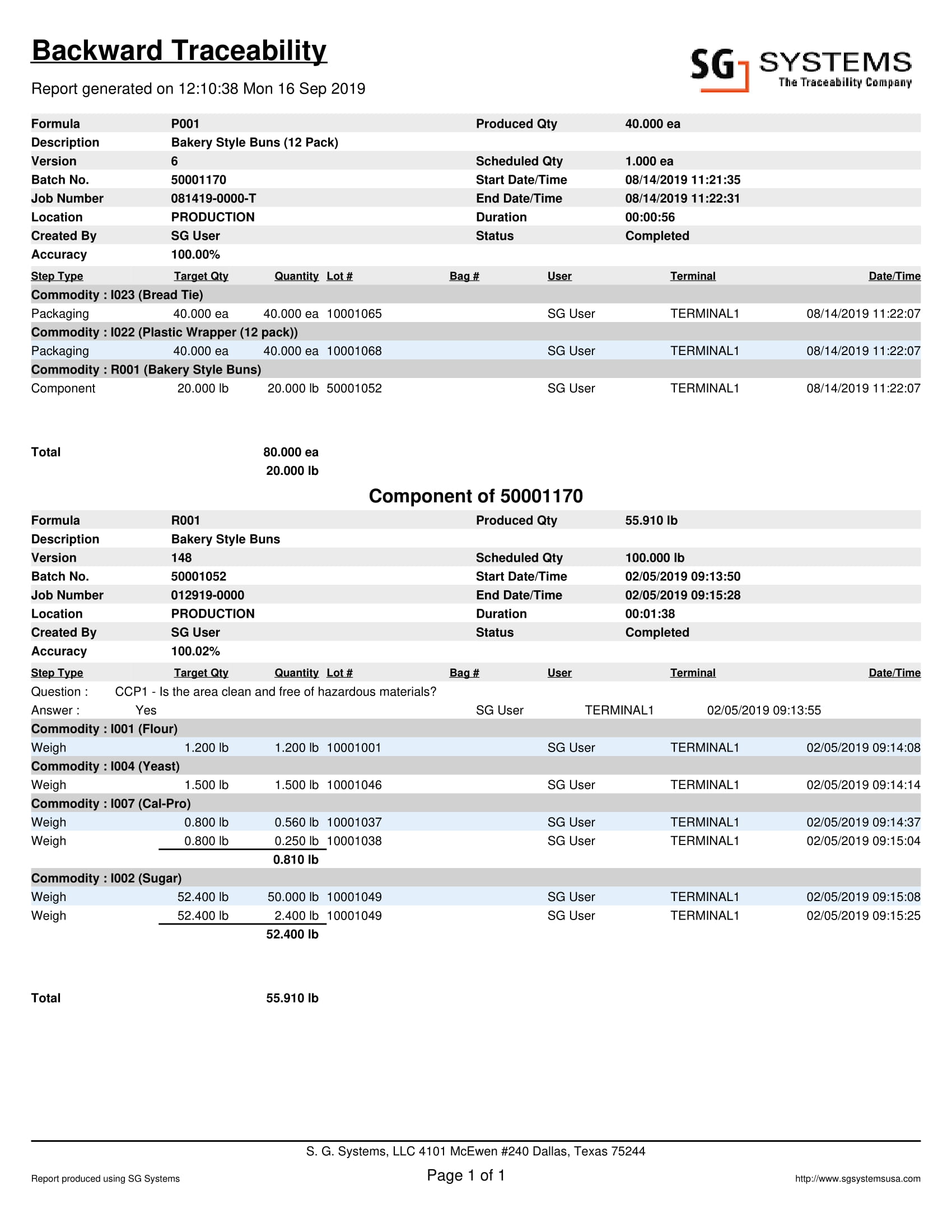
For this reason, SG Systems has developed the ‘Backward Traceability Report’. This report takes ingredient usage information from the receiving, batch / submix production, and shipping departments and links the data together in a user-friendly format. The traceback report allows the user to enter the finished goods batch/lot number, and the data will show all the manufacturing steps for the recipe with the ingredients used in the batch.
Traceback steps can include many complex manufacturing processes such as mixing, batching, blending or materials, and even the comingling of different lots (in the case of liquid ingredients within a holding tank). Integration with shop floor equipment such as scales, weighing equipment, touch screen, PLCs, SCADA systems can data capture a reality.
All the batch steps are date & time stamped, with location, lot, and ingredient usage (plus other optional information). The unique manufacturing execution and traceback system removes the administration from the exercise and gives you complete peace of mind to act quickly and responsively if and when you need to.
Performing Traceback Demonstrations to Prospects
Electronic Traceability Systems are often showcased by food manufacturing companies when prospecting and showing the capability to new potential accounts – in particular for co-manufacturing partners. There’s nothing more impressive than being able to ask your prospect to single out an ingredient from the warehouse and then perform a 100% electronic traceback exercise within 5 seconds. A traceback and without risk or embarrassment or easily identifiable errors.
What does the Backward Traceability Report Tell Us?
Forward Traceability Report sample here. The example below allows a food manufacturing company to see the final batch number (usually printed on a label and adhered to the case, but it could be an inkjet print). All the credentials about when the product was made, the accuracy of the batch (often used or yielded), the start and end time (pallet start to complete usually), plus all the information relating to the ingredient lots and usage accuracy. Information about the user and location is always helpful too.
If a batch contained a submix component, the submix would list out all the ingredients contained within and their lots used. Additional information can include quality assurance data collected along the (Trailer inspection logs, visual product inspection, put away observations, HACCP and SOP during the batch assembly phase, line inspection / metal detection results etc) way and any high-resolution images taken or uploaded about the received lots, COA’s, bill of lading, inspection logs and so on. The Backward Traceability report can be used as an electronic batch record for pharmaceutical manufacturing companies looking to achieve 21 CFR Part 11 technical compliance with a computerized system.
What is Traceability? Need some background information on the subject to increase your awareness? Click here or call our offices on 214 819 9570 to schedule a consultation to understand the options available to you within your business process. SG Systems specializes in complex shop floor manufacturing traceability and serves companies of all sizes (from smaller, single site owner managed operations all the way through to fortune 100 multinational operations and everything in between). V5 Traceability is a highly configurable and scalable solution, made to fit most applications and budgets alike. The solution can be on premised deploys or hosted within the cloud on remote servers, depending on your business data security policies.
Contacts SG Systems today to explore the V5 Traceability Solution info@v5traceability.com
North America
SG Systems LLC
PO BOX 670056, DALLAS, TX 75367-0056
Phone: +1 214 819 9570
Contact us here
United Kingdom
SG Systems Europe Ltd
Suite 3 Walton Summit Centre, Green Place, Four Oaks Rd, Preston, PR5 8AY
Phone: +44 (0) 114 349 1480
Contact us here
Australia
Wedderburn
101 Williamson Road, Ingleburn NSW 2565
Phone: +61 2 9426 1800
Contact us here
Europe
SG Traceability Systems Ltd
31-32 Greenmount Office Park, Harolds Cross, Dublin D6, Ireland
Phone: +44 (0) 114 349 1480
Contact us here
Rest assured, we value your privacy and are committed to keeping your data safe. By clicking "Continue with recommended settings," you're giving us permission to use cookies. Of course, you're in control – feel free to adjust your cookie settings anytime in our Privacy Preferences.
